Identification of Susceptibility Genes in Castanea sativa and Their Transcription Dynamics following Pathogen Infection
- PMID: 34063239
- PMCID: PMC8147476
- DOI: 10.3390/plants10050913
Identification of Susceptibility Genes in Castanea sativa and Their Transcription Dynamics following Pathogen Infection
Abstract
Castanea sativa is one of the main multipurpose tree species valued for its timber and nuts. This species is susceptible to two major diseases, ink disease and chestnut blight, caused by Phytophthora spp. and Cryphonectria parasitica, respectively. The loss-of-function mutations of genes required for the onset of pathogenesis, referred to as plant susceptibility (S) genes, are one mechanism of plant resistance against pathogens. On the basis of sequence homology, functional domain identification, and phylogenetic analyses, we report for the first time on the identification of S-genes (mlo1, dmr6, dnd1, and pmr4) in the Castanea genus. The expression dynamics of S-genes were assessed in C. sativa and C. crenata plants inoculated with P. cinnamomi and C. parasitica. Our results highlighted the upregulation of pmr4 and dmr6 in response to pathogen infection. Pmr4 was strongly expressed at early infection phases of both pathogens in C. sativa, whereas in C. crenata, no significant upregulation was observed. The infection of P. cinnamomi led to a higher increase in the transcript level of dmr6 in C. sativa compared to C. crenata-infected samples. For a better understanding of plant responses, the transcript levels of defense genes gluB and chi3 were also analyzed.
Keywords: Cryphonectria parasitica; Phytophthora cinnamomi; chestnut; susceptibility genes.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
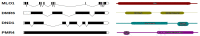






References
-
- Torello Marinoni D., Nishio S., Valentini N., Shirasawa K., Acquadro A., Portis E., Alma A., Akkak A., Pavese V., Cavalet-Giorsa E., et al. Development of High-Density Genetic Linkage Maps and Identification of Loci for Chestnut Gall Wasp Resistance in Castanea spp. Plants. 2020;9:1048. doi: 10.3390/plants9081048. - DOI - PMC - PubMed
-
- Conedera M., Krebs P., Tinner W., Pradella M., Torriani D. The cultivation of Castanea sativa (Mill.) in Europe, from its origin to its diffusion on a continental scale. Veg. Hist. Archaeobot. 2004;13:161–179. doi: 10.1007/s00334-004-0038-7. - DOI
-
- Zentmyer G.A. Phytophthora cinnamomi and The Diseases it Causes. Volume 10. The American Phytopathological Society; St. Paul, MN, USA: 1980. p. 96.
-
- Cristinzio G., Grassi G. Valutazione di resistenza a Phytophthora cambivora e Phytophthora cinnamomi in cultivar di Castanea sativa. Monti Boschi. 1993;1:54–58.
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

