Design and Optimization of Orally Administered Luteolin Nanoethosomes to Enhance Its Anti-Tumor Activity against Hepatocellular Carcinoma
- PMID: 34063274
- PMCID: PMC8147467
- DOI: 10.3390/pharmaceutics13050648
Design and Optimization of Orally Administered Luteolin Nanoethosomes to Enhance Its Anti-Tumor Activity against Hepatocellular Carcinoma
Abstract
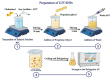
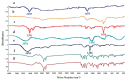
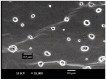
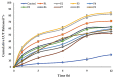
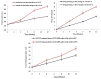
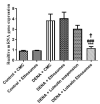
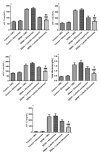
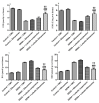
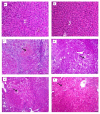
Luteolin (LUT) is a natural flavonoid with low oral bioavailability with restricted clinical applications due to its low solubility. LUT shows significant anti-tumor activity in many cancer cells, including hepatocellular carcinoma (HCC). The most recent trend in pharmaceutical innovations is the application of phospholipid vesicles to improve the solubility of such hydrophobic drugs. Ethosomes are one of the most powerful phospholipid vesicles used to achieve that that target. In this study, LUT-loaded ethosomal nanoparticles (LUT-ENPs) were prepared by the cold method. Full factorial design and response surface methodology were used to analyze and optimize the selected formulation variables. Drug entrapment efficiency, vesicle size, zeta potential, Fourier transform infra-red spectroscopy, scanning electron microscopy, and cumulative percent drug released was estimated. The selected LUT-ENPs were subjected to further investigations as estimation of hepatic gene expression levels of GPC3, liver biomarkers, and oxidative stress biomarkers. The prepared LUT-ENPs were semi-spherical in shape with high entrapment efficiency. The prepared LUT-ENPs have a small particle size with high zeta potential values. The in vitro liver biomarkers assay revealed a significant decrease in the hepatic tissue nitric oxide (NO), malondialdehyde (MDA) content, and the expression of the GPC3 gene. Results showed a high increase in the hepatic tissue levels of glutathione (GSH) and superoxide dismutase (SOD). Histopathological examination showed a small number of hepatic adenomas and a significant decrease of neoplastic hepatic lesions after treatment with LUT-ENPs. Our results firmly suggest the distinctive anti-proliferative activity of LUT-ENPs as an oral drug delivery system for the treatment of HCC.
Keywords: ethosomes; hepatocellular carcinoma; luteolin; nano-sized vesicles; nanoparticle; oxidative stress biomarkers.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Elsayed M.M., Mostafa M.E., Alaaeldin E., Sarhan H.A., Shaykoon M.S., Allam S., Ahmed A.R., Elsadek B.E. Design and characterisation of novel Sorafenib-loaded carbon nanotubes with distinct tumour-suppressive activity in hepatocellular carcinoma. Int. J. Nanomed. 2019;14:8445. doi: 10.2147/IJN.S223920. - DOI - PMC - PubMed
-
- Refaat H., Naguib Y.W., Elsayed M., Sarhan H.A., Alaaeldin E. Modified spraying technique and response surface methodology for the preparation and optimization of propolis liposomes of enhanced anti-proliferative activity against human melanoma cell line A375. Pharmaceutics. 2019;11:558. doi: 10.3390/pharmaceutics11110558. - DOI - PMC - PubMed
-
- Saddik M.S., Elsayed M., Abdelkader M.S.A., El-Mokhtar M.A., Abdel-Aleem J.A., Abu-Dief A.M., Al-Hakkani M.F., Farghaly H.S., Abou-Taleb H.A. Novel green biosynthesis of 5-fluorouracil chromium nanoparticles using harpullia pendula extract for treatment of colorectal cancer. Pharmaceutics. 2021;13:226. doi: 10.3390/pharmaceutics13020226. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

