Supramolecular Control of Singlet Oxygen Generation
- PMID: 34063309
- PMCID: PMC8124681
- DOI: 10.3390/molecules26092673
Supramolecular Control of Singlet Oxygen Generation
Abstract
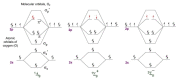
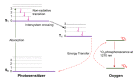
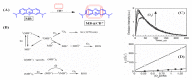
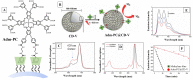
Singlet oxygen (1O2) is the excited state electronic isomer and a reactive form of molecular oxygen, which is most efficiently produced through the photosensitized excitation of ambient triplet oxygen. Photochemical singlet oxygen generation (SOG) has received tremendous attention historically, both for its practical application as well as for the fundamental aspects of its reactivity. Applications of singlet oxygen in medicine, wastewater treatment, microbial disinfection, and synthetic chemistry are the direct results of active past research into this reaction. Such advancements were achieved through design factors focused predominantly on the photosensitizer (PS), whose photoactivity is relegated to self-regulated structure and energetics in ground and excited states. However, the relatively new supramolecular approach of dictating molecular structure through non-bonding interactions has allowed photochemists to render otherwise inactive or less effective PSs as efficient 1O2 generators. This concise and first of its kind review aims to compile progress in SOG research achieved through supramolecular photochemistry in an effort to serve as a reference for future research in this direction. The aim of this review is to highlight the value in the supramolecular photochemistry approach to tapping the unexploited technological potential within this historic reaction.
Keywords: calixarene; cavitands; cucurbituril; cyclodextrin; near IR (NIR); oxidation; photodynamic therapy (PDT); photosensitizer (PS); singlet oxygen; singlet oxygen generation (SOG); supramolecular chemistry.
Conflict of interest statement
The authors declare no conflict of interest.
Figures























References
-
- Turro N.J. Modern Molecular Photochemistry. Addison-Wesley Publishing Co.; Boston, MA, USA: 1978. p. 628.
-
- Wayne R.P. Singlet molecular oxygen. Advan. Photochem. 1969;7:311–371.
-
- Krumova K., Cosa G. Overview of reactive oxygen species. Compr. Ser. Photochem. Photobiol. Sci. 2016;13:3–21.
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

