Electrochemical Biosensors in Food Safety: Challenges and Perspectives
- PMID: 34063344
- PMCID: PMC8156954
- DOI: 10.3390/molecules26102940
Electrochemical Biosensors in Food Safety: Challenges and Perspectives
Abstract
Safety and quality are key issues for the food industry. Consequently, there is growing demand to preserve the food chain and products against substances toxic, harmful to human health, such as contaminants, allergens, toxins, or pathogens. For this reason, it is mandatory to develop highly sensitive, reliable, rapid, and cost-effective sensing systems/devices, such as electrochemical sensors/biosensors. Generally, conventional techniques are limited by long analyses, expensive and complex procedures, and skilled personnel. Therefore, developing performant electrochemical biosensors can significantly support the screening of food chains and products. Here, we report some of the recent developments in this area and analyze the contributions produced by electrochemical biosensors in food screening and their challenges.
Keywords: antibiotics; bacteria; contaminants; electrochemical biosensors; food; pesticides; safety; toxins.
Conflict of interest statement
The author declares no conflict of interest.
Figures



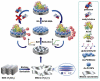
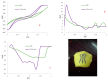


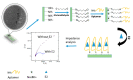
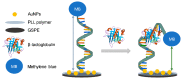
References
-
- Campuzano S., Yáñez-Sedeño P., Pingarrón J.M. Electrochemical Affinity Biosensors in Food Safety. Chemosensors. 2017;5:8. doi: 10.3390/chemosensors5010008. - DOI
-
- Scognamiglio V., Arduini F., Palleschi G., Rea G. Biosensing technology for sustainable food safety. TrAC Trends Anal. Chem. 2014;62:1–10. doi: 10.1016/j.trac.2014.07.007. - DOI
-
- Caballero B., Trugo L., Finglas P. Encyclopedia of Food Sciences and Nutrition: Volumes 1–10. Elsevier; New York, NY, USA: 2003.
-
- Pividori M.I., Alegret S. Electrochemical biosensors for food safety. Contrib. Sci. 2010;6:173–191.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

