Antibody-Drug Conjugates-A Tutorial Review
- PMID: 34063364
- PMCID: PMC8156828
- DOI: 10.3390/molecules26102943
Antibody-Drug Conjugates-A Tutorial Review
Abstract
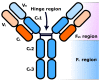
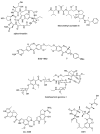
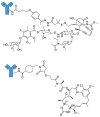
Antibody-drug conjugates (ADCs) are a family of targeted therapeutic agents for the treatment of cancer. ADC development is a rapidly expanding field of research, with over 80 ADCs currently in clinical development and eleven ADCs (nine containing small-molecule payloads and two with biological toxins) approved for use by the FDA. Compared to traditional small-molecule approaches, ADCs offer enhanced targeting of cancer cells along with reduced toxic side effects, making them an attractive prospect in the field of oncology. To this end, this tutorial review aims to serve as a reference material for ADCs and give readers a comprehensive understanding of ADCs; it explores and explains each ADC component (monoclonal antibody, linker moiety and cytotoxic payload) individually, highlights several EMA- and FDA-approved ADCs by way of case studies and offers a brief future perspective on the field of ADC research.
Keywords: ADC; antibody–drug conjugate; cytotoxic payload; linker; monoclonal antibody; tutorial review.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- How Chemotherapy Works. [(accessed on 1 October 2020)]; Available online: https://www.cancerresearchuk.org/about-cancer/cancer-in-general/treatmen....
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

