Ex Vivo Mesenchymal Stem Cell Therapy to Regenerate Machine Perfused Organs
- PMID: 34063399
- PMCID: PMC8156338
- DOI: 10.3390/ijms22105233
Ex Vivo Mesenchymal Stem Cell Therapy to Regenerate Machine Perfused Organs
Abstract
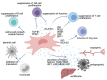
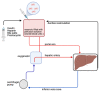
Transplantation represents the treatment of choice for many end-stage diseases but is limited by the shortage of healthy donor organs. Ex situ normothermic machine perfusion (NMP) has the potential to extend the donor pool by facilitating the use of marginal quality organs such as those from donors after cardiac death (DCD) and extended criteria donors (ECD). NMP provides a platform for organ quality assessment but also offers the opportunity to treat and eventually regenerate organs during the perfusion process prior to transplantation. Due to their anti-inflammatory, immunomodulatory and regenerative capacity, mesenchymal stem cells (MSCs) are considered as an interesting tool in this model system. Only a limited number of studies have reported on the use of MSCs during ex situ machine perfusion so far with a focus on feasibility and safety aspects. At this point, no clinical benefits have been conclusively demonstrated, and studies with controlled transplantation set-ups are urgently warranted to elucidate favorable effects of MSCs in order to improve organs during ex situ machine perfusion.
Keywords: machine perfusion; mesenchymal stem cells; regeneration.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Combination of mesenchymal stromal cells and machine perfusion is a novel strategy for organ preservation in solid organ transplantation.Cell Tissue Res. 2021 Apr;384(1):13-23. doi: 10.1007/s00441-020-03406-3. Epub 2021 Jan 13. Cell Tissue Res. 2021. PMID: 33439348 Free PMC article. Review.
-
Transplanting Marginal Organs in the Era of Modern Machine Perfusion and Advanced Organ Monitoring.Front Immunol. 2020 May 12;11:631. doi: 10.3389/fimmu.2020.00631. eCollection 2020. Front Immunol. 2020. PMID: 32477321 Free PMC article. Review.
-
Mesenchymal Stromal Cells as Anti-Inflammatory and Regenerative Mediators for Donor Kidneys During Normothermic Machine Perfusion.Stem Cells Dev. 2017 Aug 15;26(16):1162-1170. doi: 10.1089/scd.2017.0030. Epub 2017 Jun 26. Stem Cells Dev. 2017. PMID: 28557562 Review.
-
Machine perfusion in organ transplantation: a tool for ex-vivo graft conditioning with mesenchymal stem cells?Curr Opin Organ Transplant. 2013 Feb;18(1):24-33. doi: 10.1097/MOT.0b013e32835c494f. Curr Opin Organ Transplant. 2013. PMID: 23254699 Review.
-
Infusing Mesenchymal Stromal Cells into Porcine Kidneys during Normothermic Machine Perfusion: Intact MSCs Can Be Traced and Localised to Glomeruli.Int J Mol Sci. 2019 Jul 23;20(14):3607. doi: 10.3390/ijms20143607. Int J Mol Sci. 2019. PMID: 31340593 Free PMC article.
Cited by
-
Extracellular Vesicles as Drug Delivery Systems in Organ Transplantation: The Next Frontier.Pharmaceutics. 2023 Mar 9;15(3):891. doi: 10.3390/pharmaceutics15030891. Pharmaceutics. 2023. PMID: 36986753 Free PMC article. Review.
-
Dynamic conditioning of porcine kidney grafts with extracellular vesicles derived from urine progenitor cells: A proof-of-concept study.Clin Transl Med. 2024 Dec;14(12):e70095. doi: 10.1002/ctm2.70095. Clin Transl Med. 2024. PMID: 39673122 Free PMC article.
-
Long-term machine perfusion of human split livers: a new model for regenerative and translational research.Nat Commun. 2024 Nov 12;15(1):9809. doi: 10.1038/s41467-024-54024-4. Nat Commun. 2024. PMID: 39532864 Free PMC article. Review.
-
Regenerative Medicine: Role of Stem Cells and Innovative Biomaterials 2.0.Int J Mol Sci. 2022 Apr 11;23(8):4199. doi: 10.3390/ijms23084199. Int J Mol Sci. 2022. PMID: 35457017 Free PMC article.
-
Past Trends and Future Directions of Cardiac Regenerative Medicine - A Systematic Analysis of Clinical Trial Registries.J Cardiovasc Transl Res. 2025 Feb;18(1):209-220. doi: 10.1007/s12265-024-10563-1. Epub 2024 Oct 3. J Cardiovasc Transl Res. 2025. PMID: 39361114 Free PMC article.
References
-
- Eurotransplant Eurotransplant Yearly Statistics Overview 2020. [(accessed on 5 March 2021)]; Available online: https://statistics.eurotransplant.org/index.php?search_type=overview&sea....
-
- Wolfe R.A., Ashby V.B., Milford E.L., Ojo A.O., Ettenger R.E., Agodoa L.Y., Held P.J., Port F.K. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N. Engl. J. Med. 1999;341:1725–1730. doi: 10.1056/NEJM199912023412303. - DOI - PubMed
-
- Mateo R., Cho Y., Singh G., Stapfer M., Donovan J., Kahn J., Fong T.L., Sher L., Jabbour N., Aswad S., et al. Risk factors for graft survival after liver transplantation from donation after cardiac death donors: An analysis of OPTN/UNOS data. Am. J. Transplant. 2006;6:791–796. doi: 10.1111/j.1600-6143.2006.01243.x. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

