Sympathetic Activation and Arrhythmogenesis after Myocardial Infarction: Where Do We Stand?
- PMID: 34063477
- PMCID: PMC8156099
- DOI: 10.3390/jcdd8050057
Sympathetic Activation and Arrhythmogenesis after Myocardial Infarction: Where Do We Stand?
Abstract
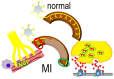
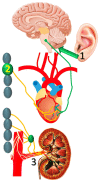
Myocardial infarction often leads to progressive structural and electrophysiologic remodeling of the left ventricle. Despite the widespread use of β-adrenergic blockade and implantable defibrillators, morbidity and mortality from chronic-phase ventricular tachyarrhythmias remains high, calling for further investigation on the underlying pathophysiology. Histological and functional studies have demonstrated extensive alterations of sympathetic nerve endings at the peri-infarct area and flow-innervation mismatches that create a highly arrhythmogenic milieu. Such accumulated evidence, along with the previously well-documented autonomic dysfunction as an important contributing factor, has stirred intense research interest for pharmacologic and non-pharmacologic neuromodulation in post-infarction heart failure. In this regard, aldosterone inhibitors, sacubitril/valsartan and sodium-glucose cotransporter type 2 inhibitors have shown antiarrhythmic effects. Non-pharmacologic modalities, currently tested in pre-clinical and clinical trials, include transcutaneous vagal stimulation, stellate ganglion modulation and renal sympathetic denervation. In this review, we provide insights on the pathophysiology of ventricular arrhythmogenesis post-myocardial infarction, focusing on sympathetic activation.
Keywords: myocardial infarction; sympathetic activation; ventricular tachyarrhythmias.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Paranskaya L., Akin I., Chatterjee T., Ritz A., Paranski P., Rehders T., Ince H., Schneider H., Nienaber C.A., Bansch D. Ventricular tachycardia and sudden death after primary PCI-reperfusion therapy: Impact on primary prevention of sudden cardiac death. Herzschrittmacherther. Elektrophysiol. 2011;22:243–248. doi: 10.1007/s00399-011-0160-z. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

