A Bittersweet Computational Journey among Glycosaminoglycans
- PMID: 34063530
- PMCID: PMC8156566
- DOI: 10.3390/biom11050739
A Bittersweet Computational Journey among Glycosaminoglycans
Abstract
Glycosaminoglycans (GAGs) are linear polysaccharides. In proteoglycans (PGs), they are attached to a core protein. GAGs and PGs can be found as free molecules, associated with the extracellular matrix or expressed on the cell membrane. They play a role in the regulation of a wide array of physiological and pathological processes by binding to different proteins, thus modulating their structure and function, and their concentration and availability in the microenvironment. Unfortunately, the enormous structural diversity of GAGs/PGs has hampered the development of dedicated analytical technologies and experimental models. Similarly, computational approaches (in particular, molecular modeling, docking and dynamics simulations) have not been fully exploited in glycobiology, despite their potential to demystify the complexity of GAGs/PGs at a structural and functional level. Here, we review the state-of-the art of computational approaches to studying GAGs/PGs with the aim of pointing out the "bitter" and "sweet" aspects of this field of research. Furthermore, we attempt to bridge the gap between bioinformatics and glycobiology, which have so far been kept apart by conceptual and technical differences. For this purpose, we provide computational scientists and glycobiologists with the fundamentals of these two fields of research, with the aim of creating opportunities for their combined exploitation, and thereby contributing to a substantial improvement in scientific knowledge.
Keywords: glycosaminoglycans; heparan sulfate; heparin; molecular docking; molecular dynamic simulations; molecular modeling.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures







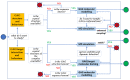
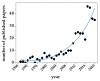
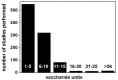



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

