Ribonucleic Acid Export 1 Is a Kinetochore-Associated Protein That Participates in Chromosome Alignment in Mouse Oocytes
- PMID: 34063622
- PMCID: PMC8125685
- DOI: 10.3390/ijms22094841
Ribonucleic Acid Export 1 Is a Kinetochore-Associated Protein That Participates in Chromosome Alignment in Mouse Oocytes
Abstract
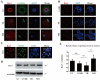
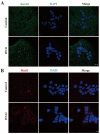
Ribonucleic acid export 1 (Rae1) is an important nucleoporin that participates in mRNA export during the interphase of higher eukaryotes and regulates the mitotic cell cycle. In this study, small RNA interference technology was used to knockdown Rae1, and immunofluorescence, immunoblotting, and chromosome spreading were used to study the role of Rae1 in mouse oocyte meiotic maturation. We found that Rae1 is a crucial regulator of meiotic maturation of mouse oocytes. After the resumption of meiosis (GVBD), Rae1 was concentrated on the kinetochore structure. The knockdown of Rae1 by a specific siRNA inhibited GVBD progression at 2 h, finally leading to a decreased 14 h polar body extrusion (PBE) rate. However, a comparable 14 h PBE rate was found in the control, and the Rae1 knockdown groups that had already undergone GVBD. Furthermore, we found elevated PBE after 9.5 h in the Rae1 knockdown oocytes. Further analysis revealed that Rae1 depletion significantly decreased the protein level of securin. In addition, we detected weakened kinetochore-microtubule (K-MT) attachments, misaligned chromosomes, and an increased incidence of aneuploidy in the Rae1 knockdown oocytes. Collectively, we propose that Rae1 modulates securin protein levels, which contribute to chromosome alignment, K-MT attachments, and aneuploidy in meiosis.
Keywords: Rae1; aneuploidy; chromosome alignment; meiotic maturation; mouse oocyte; securin.
Conflict of interest statement
The authors declare that no competing interests exist.
Figures




Similar articles
-
Nucleoporin35 is a novel microtubule associated protein functioning in oocyte meiotic spindle architecture.Exp Cell Res. 2018 Oct 15;371(2):435-443. doi: 10.1016/j.yexcr.2018.09.004. Epub 2018 Sep 5. Exp Cell Res. 2018. PMID: 30195030
-
Drosophila rae1 is required for male meiosis and spermatogenesis.J Cell Sci. 2013 Aug 15;126(Pt 16):3541-51. doi: 10.1242/jcs.111328. Epub 2013 Jun 20. J Cell Sci. 2013. PMID: 23788425
-
Zfp207 is a Bub3 binding protein regulating meiotic chromosome alignment in mouse oocytes.Oncotarget. 2016 May 24;7(21):30155-65. doi: 10.18632/oncotarget.9310. Oncotarget. 2016. PMID: 27177335 Free PMC article.
-
CDCA8 regulates meiotic spindle assembly and chromosome segregation during human oocyte meiosis.Gene. 2020 May 30;741:144495. doi: 10.1016/j.gene.2020.144495. Epub 2020 Feb 20. Gene. 2020. PMID: 32088244
-
CENP-W regulates kinetochore-microtubule attachment and meiotic progression of mouse oocytes.Biochem Biophys Res Commun. 2020 Jun 18;527(1):8-14. doi: 10.1016/j.bbrc.2020.04.078. Epub 2020 Apr 24. Biochem Biophys Res Commun. 2020. PMID: 32446395
Cited by
-
RAE1 is a prognostic biomarker and is correlated with clinicopathological characteristics of patients with hepatocellular carcinoma.BMC Bioinformatics. 2022 Jun 24;23(1):252. doi: 10.1186/s12859-022-04806-8. BMC Bioinformatics. 2022. PMID: 35751040 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

