Reactive Oxygen Species-Induced TRPM2-Mediated Ca2+ Signalling in Endothelial Cells
- PMID: 34063677
- PMCID: PMC8147627
- DOI: 10.3390/antiox10050718
Reactive Oxygen Species-Induced TRPM2-Mediated Ca2+ Signalling in Endothelial Cells
Abstract
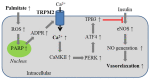
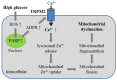
Endothelial cells form the innermost layer of blood vessels with a fundamental role as the physical barrier. While regulation of endothelial cell function by reactive oxygen species (ROS) is critical in physiological processes such as angiogenesis, endothelial function is a major target for interruption by oxidative stress resulting from generation of high levels of ROS in endothelial cells by various pathological factors and also release of ROS by neutrophils. TRPM2 is a ROS-sensitive Ca2+-permeable channel expressed in endothelial cells of various vascular beds. In this review, we provide an overview of the TRPM2 channel and its role in mediating ROS-induced Ca2+ signaling in endothelial cells. We discuss the TRPM2-mediated Ca2+ signaling in vascular endothelial growth factor-induced angiogenesis and in post-ischemic neovascularization. In particular, we examine the accumulative evidence that supports the role of TRPM2-mediated Ca2+ signaling in endothelial cell dysfunction caused by various oxidative stress-inducing factors that are associated with tissue inflammation, obesity and diabetes, as well as air pollution. These findings provide new, mechanistic insights into ROS-mediated regulation of endothelial cells in physiology and diseases.
Keywords: Ca2+ signaling; ROS; TRPM2 channel; angiogenesis; barrier dysfunction; endothelial cells; vascular diseases.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Novel role of reactive oxygen species-activated Trp melastatin channel-2 in mediating angiogenesis and postischemic neovascularization.Arterioscler Thromb Vasc Biol. 2015 Apr;35(4):877-87. doi: 10.1161/ATVBAHA.114.304802. Epub 2015 Feb 12. Arterioscler Thromb Vasc Biol. 2015. PMID: 25675998 Free PMC article.
-
TRPM2 Channel-Mediated ROS-Sensitive Ca(2+) Signaling Mechanisms in Immune Cells.Front Immunol. 2015 Aug 7;6:407. doi: 10.3389/fimmu.2015.00407. eCollection 2015. Front Immunol. 2015. PMID: 26300888 Free PMC article. Review.
-
Free fatty acid-induced H2O2 activates TRPM2 to aggravate endothelial insulin resistance via Ca2+-dependent PERK/ATF4/TRB3 cascade in obese mice.Free Radic Biol Med. 2019 Nov 1;143:288-299. doi: 10.1016/j.freeradbiomed.2019.08.018. Epub 2019 Aug 21. Free Radic Biol Med. 2019. PMID: 31445205
-
Targeting TRPM2 in ROS-Coupled Diseases.Pharmaceuticals (Basel). 2016 Sep 7;9(3):57. doi: 10.3390/ph9030057. Pharmaceuticals (Basel). 2016. PMID: 27618067 Free PMC article. Review.
-
A critical role of the transient receptor potential melastatin 2 channel in a positive feedback mechanism for reactive oxygen species-induced delayed cell death.J Cell Physiol. 2019 Apr;234(4):3647-3660. doi: 10.1002/jcp.27134. Epub 2018 Sep 19. J Cell Physiol. 2019. PMID: 30229906
Cited by
-
Endothelial Dysfunction and Diabetic Cardiomyopathy.Front Endocrinol (Lausanne). 2022 Apr 7;13:851941. doi: 10.3389/fendo.2022.851941. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 35464057 Free PMC article. Review.
-
TRPM2 Non-Selective Cation Channels in Liver Injury Mediated by Reactive Oxygen Species.Antioxidants (Basel). 2021 Aug 3;10(8):1243. doi: 10.3390/antiox10081243. Antioxidants (Basel). 2021. PMID: 34439491 Free PMC article. Review.
-
Microfluidic investigation for shear-stress-mediated repair of dysglycemia-induced endothelial cell damage.Mechanobiol Med. 2024 Apr 29;2(3):100069. doi: 10.1016/j.mbm.2024.100069. eCollection 2024 Sep. Mechanobiol Med. 2024. PMID: 40395495 Free PMC article. Review.
-
The ROS Mediates MCUb in Mitochondria-Regulated Apoptosis of TM4 Cells Induced by Titanium Dioxide Nanoparticles.Biol Trace Elem Res. 2025 May;203(5):2760-2775. doi: 10.1007/s12011-024-04339-6. Epub 2024 Aug 27. Biol Trace Elem Res. 2025. PMID: 39192169
-
TRP Channels in Tumoral Processes Mediated by Oxidative Stress and Inflammation.Antioxidants (Basel). 2023 Jun 23;12(7):1327. doi: 10.3390/antiox12071327. Antioxidants (Basel). 2023. PMID: 37507867 Free PMC article. Review.
References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

