Glucose-Limited Fed-Batch Cultivation Strategy to Mimic Large-Scale Effects in Escherichia coli Linked to Accumulation of Non-Canonical Branched-Chain Amino Acids by Combination of Pyruvate Pulses and Dissolved Oxygen Limitation
- PMID: 34063744
- PMCID: PMC8223794
- DOI: 10.3390/microorganisms9061110
Glucose-Limited Fed-Batch Cultivation Strategy to Mimic Large-Scale Effects in Escherichia coli Linked to Accumulation of Non-Canonical Branched-Chain Amino Acids by Combination of Pyruvate Pulses and Dissolved Oxygen Limitation
Abstract
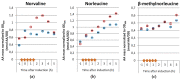
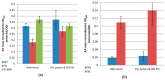
Insufficient mixing in large-scale bioreactors provokes gradient zones of substrate, dissolved oxygen (DO), pH, and other parameters. E. coli responds to a high glucose, low oxygen feeding zone with the accumulation of mixed acid fermentation products, especially formate, but also with the synthesis of non-canonical amino acids, such as norvaline, norleucine and β-methylnorleucine. These amino acids can be mis-incorporated into recombinant products, which causes a problem for pharmaceutical production whose solution is not trivial. While these effects can also be observed in scale down bioreactor systems, these are challenging to operate. Especially the high-throughput screening of clone libraries is not easy, as fed-batch cultivations would need to be controlled via repeated glucose pulses with simultaneous oxygen limitation, as has been demonstrated in well controlled robotic systems. Here we show that not only glucose pulses in combination with oxygen limitation can provoke the synthesis of these non-canonical branched-chain amino acids (ncBCAA), but also that pyruvate pulses produce the same effect. Therefore, we combined the enzyme-based glucose delivery method Enbase® in a PALL24 mini-bioreactor system and combined repeated pyruvate pulses with simultaneous reduction of the aeration rate. These cultivation conditions produced an increase in the non-canonical branched chain amino acids norvaline and norleucine in both the intracellular soluble protein and inclusion body fractions with mini-proinsulin as an example product, and this effect was verified in a 15 L stirred tank bioreactor (STR). To our opinion this cultivation strategy is easy to apply for the screening of strain libraries under standard laboratory conditions if no complex robotic and well controlled parallel cultivation devices are available.
Keywords: mixed-acid fermentation; non-canonical branched chain amino acids; pyruvate pulse; scale-down; strain screening.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures



References
-
- Bylund F., Collet E., Enfors S.O., Larsson G. Substrate gradient formation in the large-scale bioreactor lowers cell yield and increases by-product formation. Bioprocess Eng. 1998;18:171–180. doi: 10.1007/s004490050427. - DOI
-
- Hewitt C.J., Nienow A.W. The scale-up of microbial batch and fed-batch fermentation processes. Adv. Appl. Microbiol. 2007;62:105–135. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

