Systemic Treatment Initiation in Classical and Endemic Kaposi's Sarcoma: Risk Factors and Global Multi-State Modelling in a Monocentric Cohort Study
- PMID: 34063894
- PMCID: PMC8196666
- DOI: 10.3390/cancers13112519
Systemic Treatment Initiation in Classical and Endemic Kaposi's Sarcoma: Risk Factors and Global Multi-State Modelling in a Monocentric Cohort Study
Abstract
Background: Although several studies described the clinical course of epidemic and post-transplant Kaposi's Sarcoma (KS), the lack of large cohorts of classic/endemic KS, precluded such characterization.
Methods: We used multi-state modelling in a retrospective monocentric study to evaluate global disease evolution and identify risk factors for systemic treatment (ST) initiation. 160 classic/endemic KS patients consecutively diagnosed between 1990 and 2013 were included.
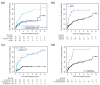
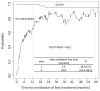
Results: 41.2% of classic/endemic KS patients required ST. Cumulative incidence of ST after 2 years of follow-up was 28.4% [95% CI: 20.5; 35.5]. Multivariate analysis identified six risk factors for ST initiation: time between first symptoms and diagnosis ≥1 year, endemic KS, total number of lesions ≥10, visceral, head or neck localization and presence of edema. Type of ST, type of KS, age and time between diagnosis and ST were not associated with response. Mean treatment-free time during the first 5 years following ST was 44 months for interferon and 44.6 months for chemotherapy treated patients (Mean difference: -0.5 months [95% CI: -9.5; 4.9]).
Conclusions: Our study reveals ST risk factors in classic/endemic KS and highlights the clinical aggressiveness of the endemic KS subtype. No efficacy difference was observed between standard of care treatments, enabling treatment choice based on patient's fitness.
Keywords: chemotherapy; classical and endemic Kaposi Sarcoma; interferon; multi-state modelling; systemic treatment; treatment free interval.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Lambert M., Gannage M., Karras A., Abel M., Legendre C., Kerob D., Agbalika F., Girard P.-M., Lebbe C., Caillat-Zucman S. Differences in the frequency and function of HHV8-specific CD8 T cells between asymptomatic HHV8 infection and Kaposi sarcoma. Blood. 2006;108:3871–3880. doi: 10.1182/blood-2006-03-014225. - DOI - PubMed
LinkOut - more resources
Full Text Sources

