Age-Related Changes in Bone-Marrow Mesenchymal Stem Cells
- PMID: 34063923
- PMCID: PMC8223980
- DOI: 10.3390/cells10061273
Age-Related Changes in Bone-Marrow Mesenchymal Stem Cells
Abstract
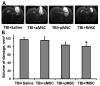
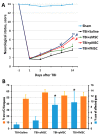
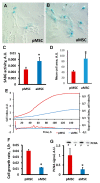
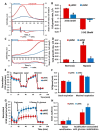
The use of stem cells is part of a strategy for the treatment of a large number of diseases. However, the source of the original stem cells for use is extremely important and determines their therapeutic potential. Mesenchymal stromal cells (MSC) have proven their therapeutic effectiveness when used in a number of pathological models. However, it remains an open question whether the chronological age of the donor organism affects the effectiveness of the use of MSC. The asymmetric division of stem cells, the result of which is some residential stem cells acquiring a non-senile phenotype, means that stem cells possess an intrinsic ability to preserve juvenile characteristics, implying an absence or at least remarkable retardation of senescence in stem cells. To test whether residential MSC senesce, we evaluated the physiological changes in the MSC from old rats, with a further comparison of the neuroprotective properties of MSC from young and old animals in a model of traumatic brain injury. We found that, while the effect of administration of MSC on lesion volume was minimal, functional recovery was remarkable, with the highest effect assigned to fetal cells; the lowest effect was recorded for cells isolated from adult rats and postnatal cells, having intermediate potency. MSC from the young rats were characterized by a faster growth than adult MSC, correlating with levels of proliferating cell nuclear antigen (PCNA). However, there were no differences in respiratory activity of MSC from young and old rats, but young cells showed much higher glucose utilization than old ones. Autophagy flux was almost the same in both types of cells, but there were remarkable ultrastructural differences in old and young cells.
Keywords: aging; glucose utilization; glycolysis; mesenchymal stromal cells; mitochondria; oxidative phosphorylation; proliferation; senescence; stem; therapy; traumatic brain injury.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Differential expression of cell cycle and WNT pathway-related genes accounts for differences in the growth and differentiation potential of Wharton's jelly and bone marrow-derived mesenchymal stem cells.Stem Cell Res Ther. 2017 Apr 26;8(1):102. doi: 10.1186/s13287-017-0555-9. Stem Cell Res Ther. 2017. PMID: 28446235 Free PMC article.
-
A number of bone marrow mesenchymal stem cells but neither phenotype nor differentiation capacities changes with age of rats.Mol Cells. 2007 Oct 31;24(2):255-60. Mol Cells. 2007. PMID: 17978579
-
Survival/Adaptation of Bone Marrow-Derived Mesenchymal Stem Cells After Long-Term Starvation Through Selective Processes.Stem Cells. 2019 Jun;37(6):813-827. doi: 10.1002/stem.2998. Epub 2019 Mar 25. Stem Cells. 2019. PMID: 30835892
-
Human stromal (mesenchymal) stem cells from bone marrow, adipose tissue and skin exhibit differences in molecular phenotype and differentiation potential.Stem Cell Rev Rep. 2013 Feb;9(1):32-43. doi: 10.1007/s12015-012-9365-8. Stem Cell Rev Rep. 2013. PMID: 22529014 Free PMC article.
-
The other side of the coin: mesenchymal stromal cell immortalization beyond evasion of senescence.Hum Cell. 2023 Sep;36(5):1593-1603. doi: 10.1007/s13577-023-00925-3. Epub 2023 Jun 21. Hum Cell. 2023. PMID: 37341871 Review.
Cited by
-
Metformin rejuvenates Nap1l2-impaired immunomodulation of bone marrow mesenchymal stem cells via metabolic reprogramming.Cell Prolif. 2024 Jul;57(7):e13612. doi: 10.1111/cpr.13612. Epub 2024 Feb 13. Cell Prolif. 2024. PMID: 38348888 Free PMC article.
-
Mesenchymal stem cell-based therapy for paraquat-induced lung injury.Cell Biol Toxicol. 2024 Aug 13;40(1):70. doi: 10.1007/s10565-024-09911-3. Cell Biol Toxicol. 2024. PMID: 39136896 Free PMC article. Review.
-
A Review of the Use of Extracellular Vesicles in the Treatment of Neonatal Diseases: Current State and Problems with Translation to the Clinic.Int J Mol Sci. 2024 Mar 1;25(5):2879. doi: 10.3390/ijms25052879. Int J Mol Sci. 2024. PMID: 38474125 Free PMC article. Review.
-
Variant load of mitochondrial DNA in single human mesenchymal stem cells.Sci Rep. 2024 Sep 9;14(1):20989. doi: 10.1038/s41598-024-71822-4. Sci Rep. 2024. PMID: 39251776 Free PMC article.
-
MiR-34a-HK1 signal axis retards bone marrow mesenchymal stem cell senescence via ameliorating glycolytic metabolism.Stem Cell Res Ther. 2024 Jul 30;15(1):238. doi: 10.1186/s13287-024-03857-3. Stem Cell Res Ther. 2024. PMID: 39080798 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

