Myasthenia Gravis: Epidemiology, Pathophysiology and Clinical Manifestations
- PMID: 34064035
- PMCID: PMC8196750
- DOI: 10.3390/jcm10112235
Myasthenia Gravis: Epidemiology, Pathophysiology and Clinical Manifestations
Abstract
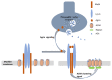
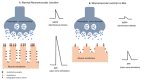
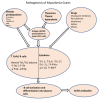
Myasthenia gravis (MG) is an autoimmune neurological disorder characterized by defective transmission at the neuromuscular junction. The incidence of the disease is 4.1 to 30 cases per million person-years, and the prevalence rate ranges from 150 to 200 cases per million. MG is considered a classic example of antibody-mediated autoimmune disease. Most patients with MG have autoantibodies against the acetylcholine receptors (AChRs). Less commonly identified autoantibodies include those targeted to muscle-specific kinase (MuSK), low-density lipoprotein receptor-related protein 4 (Lrp4), and agrin. These autoantibodies disrupt cholinergic transmission between nerve terminals and muscle fibers by causing downregulation, destruction, functional blocking of AChRs, or disrupting the clustering of AChRs in the postsynaptic membrane. The core clinical manifestation of MG is fatigable muscle weakness, which may affect ocular, bulbar, respiratory and limb muscles. Clinical manifestations vary according to the type of autoantibody, and whether a thymoma is present.
Keywords: B cells; T cells; acetylcholine receptor; autoantibodies; cytokines; myasthenia gravis.
Conflict of interest statement
K.R. has received honoraria for consultations and serving on advisory boards for Alexion, Kabafusion and Grifols. B.S. and K.R. have received funding from Alexion for conducting clinical trials on MG and ALS. L.D. and R.W. declare no conflict of interest.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

