Near Infrared Photoimmunotherapy; A Review of Targets for Cancer Therapy
- PMID: 34064074
- PMCID: PMC8196790
- DOI: 10.3390/cancers13112535
Near Infrared Photoimmunotherapy; A Review of Targets for Cancer Therapy
Abstract
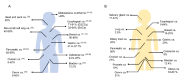
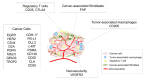
Near-infrared photoimmunotherapy (NIR-PIT) is a newly developed cancer treatment that uses an antibody-photoabsorber (IRDye700DX) conjugate (APC) that is activated by NIR light irradiation. In September 2020, the first APC and laser system were conditionally approved for clinical use in Japan. A major benefit of NIR-PIT is that only APC-bound cancer cells that are exposed to NIR light are killed by NIR-PIT; thus, minimal damage occurs in adjacent normal cells. These early trials have demonstrated that in addition to direct cell killing, there is a significant therapeutic host immune response that greatly contributes to the success of the therapy. Although the first clinical use of NIR-PIT targeted epidermal growth factor receptor (EGFR), many other targets are suitable for NIR-PIT. NIR-PIT has now been applied to many cancers expressing various cell-surface target proteins using monoclonal antibodies designed to bind to them. Moreover, NIR-PIT is not limited to tumor antigens but can also be used to kill specific host cells that create immune-permissive environments in which tumors grow. Moreover, multiple targets can be treated simultaneously with NIR-PIT using a cocktail of APCs. NIR-PIT can be used in combination with other therapies, such as immune checkpoint inhibitors, to enhance the therapeutic effect. Thus, NIR-PIT has great potential to treat a wide variety of cancers by targeting appropriate tumor cells, immune cells, or both, and can be augmented by other immunotherapies.
Keywords: cancer; cancer therapy; host immunity; near-infrared photoimmunotherapy (NIR-PIT); target molecule.
Conflict of interest statement
The authors have no conflict of interest to disclose.
Figures

Similar articles
-
Near-Infrared Photoimmunotherapy of Cancer.Acc Chem Res. 2019 Aug 20;52(8):2332-2339. doi: 10.1021/acs.accounts.9b00273. Epub 2019 Jul 23. Acc Chem Res. 2019. PMID: 31335117 Free PMC article. Review.
-
Near infrared photoimmunotherapy of cancer; possible clinical applications.Nanophotonics. 2021 May 7;10(12):3135-3151. doi: 10.1515/nanoph-2021-0119. eCollection 2021 Sep. Nanophotonics. 2021. PMID: 36405499 Free PMC article. Review.
-
Near-Infrared Photoimmunotherapy (NIR-PIT) in Urologic Cancers.Cancers (Basel). 2022 Jun 17;14(12):2996. doi: 10.3390/cancers14122996. Cancers (Basel). 2022. PMID: 35740662 Free PMC article. Review.
-
Near-infrared photoimmunotherapy of cancer: a new approach that kills cancer cells and enhances anti-cancer host immunity.Int Immunol. 2021 Jan 1;33(1):7-15. doi: 10.1093/intimm/dxaa037. Int Immunol. 2021. PMID: 32496557 Free PMC article.
-
Antibody Drug Conjugates of Near-Infrared Photoimmunotherapy (NIR-PIT) in Breast Cancers.Technol Cancer Res Treat. 2023 Jan-Dec;22:15330338221145992. doi: 10.1177/15330338221145992. Technol Cancer Res Treat. 2023. PMID: 36734039 Free PMC article. Review.
Cited by
-
Elimination of radiation-induced senescent cancer cells and stromal cells in vitro by near-infrared photoimmunotherapy.Cancer Med. 2024 Jun;13(12):e7381. doi: 10.1002/cam4.7381. Cancer Med. 2024. PMID: 38888415 Free PMC article.
-
EGFR-Targeted Photodynamic Therapy.Pharmaceutics. 2022 Jan 20;14(2):241. doi: 10.3390/pharmaceutics14020241. Pharmaceutics. 2022. PMID: 35213974 Free PMC article. Review.
-
Comparison of the Effectiveness of IgG Antibody versus F(ab')2 Antibody Fragment in CTLA4-Targeted Near-Infrared Photoimmunotherapy.Mol Pharm. 2022 Oct 3;19(10):3600-3611. doi: 10.1021/acs.molpharmaceut.2c00242. Epub 2022 Jun 27. Mol Pharm. 2022. PMID: 35759343 Free PMC article.
-
Opening up new VISTAs: V-domain immunoglobulin suppressor of T cell activation (VISTA) targeted near-infrared photoimmunotherapy (NIR-PIT) for enhancing host immunity against cancers.Cancer Immunol Immunother. 2022 Dec;71(12):2869-2879. doi: 10.1007/s00262-022-03205-5. Epub 2022 Apr 21. Cancer Immunol Immunother. 2022. PMID: 35445836 Free PMC article.
-
Simultaneously Combined Cancer Cell- and CTLA4-Targeted NIR-PIT Causes a Synergistic Treatment Effect in Syngeneic Mouse Models.Mol Cancer Ther. 2021 Nov;20(11):2262-2273. doi: 10.1158/1535-7163.MCT-21-0470. Epub 2021 Sep 13. Mol Cancer Ther. 2021. PMID: 34518299 Free PMC article.
References
-
- Mew D., Wat C.K., Towers G.H., Levy J.G. Photoimmunotherapy: Treatment of animal tumors with tumor-specific mono-clonal antibody-hematoporphyrin conjugates. J. Immunol. 1983;130:1473–1477. - PubMed
-
- Lin C.W., Amano T., Rutledge A.R., Shulok J.R., Prout G.R. Photodynamic effect in an experimental bladder tumor treated with intratumor injection of hematoporphyrin derivative. Cancer Res. 1988;48:6115–6120. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

