The Resistance Responses of Potato Plants to Potato Virus Y Are Associated with an Increased Cellular Methionine Content and an Altered SAM:SAH Methylation Index
- PMID: 34064103
- PMCID: PMC8224460
- DOI: 10.3390/v13060955
The Resistance Responses of Potato Plants to Potato Virus Y Are Associated with an Increased Cellular Methionine Content and an Altered SAM:SAH Methylation Index
Abstract
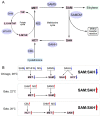
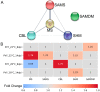
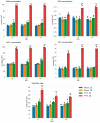
Plant-virus interactions are frequently influenced by elevated temperature, which often increases susceptibility to a virus, a scenario described for potato cultivar Chicago infected with potato virus Y (PVY). In contrast, other potato cultivars such as Gala may have similar resistances to PVY at both normal (22 °C) and high (28 °C) temperatures. To elucidate the mechanisms of temperature-independent antivirus resistance in potato, we analysed responses of Gala plants to PVY at different temperatures using proteomic, transcriptional and metabolic approaches. Here we show that in Gala, PVY infection generally upregulates the accumulation of major enzymes associated with the methionine cycle (MTC) independently of temperature, but that temperature (22 °C or 28 °C) may finely regulate what classes accumulate. The different sets of MTC-related enzymes that are up-regulated at 22 °C or 28 °C likely account for the significantly increased accumulation of S-adenosyl methionine (SAM), a key component of MTC which acts as a universal methyl donor in methylation reactions. In contrast to this, we found that in cultivar Chicago, SAM levels were significantly reduced which correlated with the enhanced susceptibility to PVY at high temperature. Collectively, these data suggest that MTC and its major transmethylation function determines resistance or susceptibility to PVY.
Keywords: isobaric tags for relative and absolute quantitation (iTRAQ); methionine cycle; plant virus resistance; potato virus Y.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Role of the methionine cycle in the temperature-sensitive responses of potato plants to potato virus Y.Mol Plant Pathol. 2021 Jan;22(1):77-91. doi: 10.1111/mpp.13009. Epub 2020 Nov 4. Mol Plant Pathol. 2021. PMID: 33146443 Free PMC article.
-
The Expression of Potato Expansin A3 (StEXPA3) and Extensin4 (StEXT4) Genes with Distribution of StEXPAs and HRGPs-Extensin Changes as an Effect of Cell Wall Rebuilding in Two Types of PVYNTN-Solanum tuberosum Interactions.Viruses. 2020 Jan 5;12(1):66. doi: 10.3390/v12010066. Viruses. 2020. PMID: 31948116 Free PMC article.
-
Streptomyces fradiae Mitigates the Impact of Potato Virus Y by Inducing Systemic Resistance in Two Egyptian Potato (Solanum tuberosum L.) Cultivars.Microb Ecol. 2024 Oct 17;87(1):131. doi: 10.1007/s00248-024-02437-5. Microb Ecol. 2024. PMID: 39419884 Free PMC article.
-
Continuous and emerging challenges of Potato virus Y in potato.Annu Rev Phytopathol. 2013;51:571-86. doi: 10.1146/annurev-phyto-082712-102332. Annu Rev Phytopathol. 2013. PMID: 23915135 Review.
-
Potato Virus Y Emergence and Evolution from the Andes of South America to Become a Major Destructive Pathogen of Potato and Other Solanaceous Crops Worldwide.Viruses. 2020 Dec 12;12(12):1430. doi: 10.3390/v12121430. Viruses. 2020. PMID: 33322703 Free PMC article. Review.
Cited by
-
Plant homocysteine, a methionine precursor and plant's hallmark of metabolic disorders.Front Plant Sci. 2022 Dec 8;13:1044944. doi: 10.3389/fpls.2022.1044944. eCollection 2022. Front Plant Sci. 2022. PMID: 36570932 Free PMC article. Review.
-
Effects of Poty-Potexvirus Synergism on Growth, Photosynthesis and Metabolite Status of Nicotiana benthamiana.Viruses. 2022 Dec 30;15(1):121. doi: 10.3390/v15010121. Viruses. 2022. PMID: 36680161 Free PMC article.
-
Leaf Bacteriome in Sugar Beet Shows Differential Response against Beet curly top virus during Resistant and Susceptible Interactions.Int J Mol Sci. 2022 Jul 22;23(15):8073. doi: 10.3390/ijms23158073. Int J Mol Sci. 2022. PMID: 35897649 Free PMC article.
-
Quantitative proteomic dataset of the moss Physcomitrium patens PSEP3 KO and OE mutant lines.Data Brief. 2021 Dec 16;40:107715. doi: 10.1016/j.dib.2021.107715. eCollection 2022 Feb. Data Brief. 2021. PMID: 34977300 Free PMC article.
-
Transcriptomic Reprogramming, Alternative Splicing and RNA Methylation in Potato (Solanum tuberosum L.) Plants in Response to Potato Virus Y Infection.Plants (Basel). 2022 Feb 25;11(5):635. doi: 10.3390/plants11050635. Plants (Basel). 2022. PMID: 35270104 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

