Regulation and Role of Transcription Factors in Osteogenesis
- PMID: 34064134
- PMCID: PMC8196788
- DOI: 10.3390/ijms22115445
Regulation and Role of Transcription Factors in Osteogenesis
Abstract
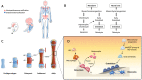
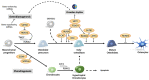
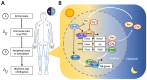
Bone is a dynamic tissue constantly responding to environmental changes such as nutritional and mechanical stress. Bone homeostasis in adult life is maintained through bone remodeling, a controlled and balanced process between bone-resorbing osteoclasts and bone-forming osteoblasts. Osteoblasts secrete matrix, with some being buried within the newly formed bone, and differentiate to osteocytes. During embryogenesis, bones are formed through intramembraneous or endochondral ossification. The former involves a direct differentiation of mesenchymal progenitor to osteoblasts, and the latter is through a cartilage template that is subsequently converted to bone. Advances in lineage tracing, cell sorting, and single-cell transcriptome studies have enabled new discoveries of gene regulation, and new populations of skeletal stem cells in multiple niches, including the cartilage growth plate, chondro-osseous junction, bone, and bone marrow, in embryonic development and postnatal life. Osteoblast differentiation is regulated by a master transcription factor RUNX2 and other factors such as OSX/SP7 and ATF4. Developmental and environmental cues affect the transcriptional activities of osteoblasts from lineage commitment to differentiation at multiple levels, fine-tuned with the involvement of co-factors, microRNAs, epigenetics, systemic factors, circadian rhythm, and the microenvironments. In this review, we will discuss these topics in relation to transcriptional controls in osteogenesis.
Keywords: bone; circadian rhythm; epigenetics; microRNA; osteoblast; osteoblast differentiation; osteogenesis; skeletogenesis; transcription factor.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Quarto N., Wan D.C., Kwan M.D., Panetta N.J., Li S., Longaker M.T. Origin matters: Differences in embryonic tissue origin and Wnt signaling determine the osteogenic potential and healing capacity of frontal and parietal calvarial bones. J. Bone Miner. Res. 2010;25:1680–1694. doi: 10.1359/jbmr.091116. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

