Anti-Angiogenic Property of Free Human Oligosaccharides
- PMID: 34064180
- PMCID: PMC8224327
- DOI: 10.3390/biom11060775
Anti-Angiogenic Property of Free Human Oligosaccharides
Abstract
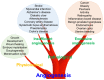
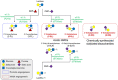
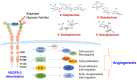
Angiogenesis, a fundamental process in human physiology and pathology, has attracted considerable attention owing to its potential as a therapeutic strategy. Vascular endothelial growth factor (VEGF) and its receptor (VEGFR) are deemed major mediators of angiogenesis. To date, inhibition of the VEGF-A/VEGFR-2 axis has been an effective strategy employed in the development of anticancer drugs. However, some limitations, such as low efficacy and side effects, need to be addressed. Several drug candidates have been discovered, including small molecule compounds, recombinant proteins, and oligosaccharides. In this review, we focus on human oligosaccharides as modulators of angiogenesis. In particular, sialylated human milk oligosaccharides (HMOs) play a significant role in the inhibition of VEGFR-2-mediated angiogenesis. We discuss the structural features concerning the interaction between sialylated HMOs and VEGFR-2 as a molecular mechanism of anti-angiogenesis modulation and its effectiveness in vivo experiments. In the current state, extensive clinical trials are required to develop a novel VEGFR-2 inhibitor from sialylated HMOs.
Keywords: VEGFR-2; angiogenesis; human milk oligosaccharides; inhibitor; sialyllactose.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

