Combined Single Nucleotide Variants of ORAI1 and BLK in a Child with Refractory Kawasaki Disease
- PMID: 34064199
- PMCID: PMC8224368
- DOI: 10.3390/children8060433
Combined Single Nucleotide Variants of ORAI1 and BLK in a Child with Refractory Kawasaki Disease
Abstract
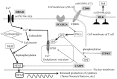
Kawasaki disease (KD) is a systemic vasculitis with an unknown etiology affecting young children. Although intravenous immunoglobulin (IVIG) plus acetylsalicylic acid is effective in most cases, approximately 10-20% of patients do not respond to this therapy. An 8-month-old boy was admitted to a local hospital with the presumptive diagnosis of KD. He received IVIG twice and four series of methylprednisolone pulse therapy from the third to the tenth day of illness. Despite these treatments, his fever persisted with the development of moderate dilatations of the coronary arteries. A diagnosis of refractory KD was made, and infliximab with oral prednisolone was administered without success. Defervescence was finally achieved by cyclosporine A, an inhibitor of the signaling pathway of the calcineurin/nuclear factor of activated T cells (NFAT). Whole-genome sequencing of his deoxyribonucleic acid samples disclosed two single nucleotide variants (SNVs) in disease-susceptibility genes in Japanese KD patients, ORAI1 (rs3741596) and BLK (rs2254546). In summary, the refractory nature of the present case could be explained by the presence of combined SNVs in susceptibility genes associated with upregulation of the calcineurin/NFAT signaling pathway. It may provide insights for stratifying KD patients based on the SNVs in their susceptibility genes.
Keywords: BLK; ORAI1; cyclosporine A; refractory Kawasaki disease; single nucleotide variant.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Steroid pulse therapy for Kawasaki disease unresponsive to additional immunoglobulin therapy.Paediatr Child Health. 2011 Oct;16(8):479-84. doi: 10.1093/pch/16.8.479. Paediatr Child Health. 2011. PMID: 23024586 Free PMC article.
-
Variations in ORAI1 Gene Associated with Kawasaki Disease.PLoS One. 2016 Jan 20;11(1):e0145486. doi: 10.1371/journal.pone.0145486. eCollection 2016. PLoS One. 2016. PMID: 26789410 Free PMC article.
-
A case of intravenous immunoglobulin-resistant Kawasaki disease treated with methotrexate.Yonsei Med J. 2002 Aug;43(4):527-32. doi: 10.3349/ymj.2002.43.4.527. Yonsei Med J. 2002. PMID: 12205742
-
Kawasaki disease: a comprehensive review of treatment options.J Clin Pharm Ther. 2015 Dec;40(6):620-5. doi: 10.1111/jcpt.12334. Epub 2015 Nov 7. J Clin Pharm Ther. 2015. PMID: 26547265 Review.
-
Comparison of second-line therapy in IVIg-refractory Kawasaki disease: a systematic review.Pediatr Rheumatol Online J. 2019 Nov 27;17(1):77. doi: 10.1186/s12969-019-0380-z. Pediatr Rheumatol Online J. 2019. PMID: 31775898 Free PMC article.
References
-
- McCrindle B.W., Rowley A.H., Newburger J.W., Burns J.C., Bolger A.F., Gewitz M., Baker A.L., Jackson M.A., Takahashi M., Shah P.B., et al. Diagnosis, treatment, and long-term management of Kawasaki disease: A scientific statement for health professionals from the American Heart Association. Circulation. 2017;135:927–999. doi: 10.1161/CIR.0000000000000484. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

