Solubilization of Trans-Resveratrol in Some Mono-Solvents and Various Propylene Glycol + Water Mixtures
- PMID: 34064283
- PMCID: PMC8196874
- DOI: 10.3390/molecules26113091
Solubilization of Trans-Resveratrol in Some Mono-Solvents and Various Propylene Glycol + Water Mixtures
Abstract
This research deals with the determination of solubility, Hansen solubility parameters, dissolution properties, enthalpy-entropy compensation, and computational modeling of a naturally-derived bioactive compound trans-resveratrol (TRV) in water, methanol, ethanol, n-propanol, n-butanol, propylene glycol (PG), and various PG + water mixtures. The solubility of TRV in six different mono-solvents and various PG + water mixtures was determined at 298.2-318.2 K and 0.1 MPa. The measured experimental solubility values of TRV were regressed using six different computational/theoretical models, including van't Hoff, Apelblat, Buchowski-Ksiazczak λh, Yalkowsly-Roseman, Jouyban-Acree, and van't Hoff-Jouyban-Acree models, with average uncertainties of less than 3.0%. The maxima of TRV solubility in mole fraction was obtained in neat PG (2.62 × 10-2) at 318.2 K. However, the minima of TRV solubility in the mole fraction was recorded in neat water (3.12 × 10-6) at 298.2 K. Thermodynamic calculation of TRV dissolution properties suggested an endothermic and entropy-driven dissolution of TRV in all studied mono-solvents and various PG + water mixtures. Solvation behavior evaluation indicated an enthalpy-driven mechanism as the main mechanism for TRV solvation. Based on these data and observations, PG has been chosen as the best mono-solvent for TRV solubilization.
Keywords: Hansen solubility parameters; computational modeling; dissolution properties; solubility; trans-resveratrol.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

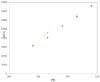
 represents the experimental TRV solubility in neat water; the symbol
represents the experimental TRV solubility in neat water; the symbol  represents the reported solubility values of TRV in neat water taken from reference [27]; the symbol
represents the reported solubility values of TRV in neat water taken from reference [27]; the symbol  represents the reported solubility values of TRV in neat water taken from reference [1].
represents the reported solubility values of TRV in neat water taken from reference [1].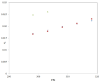
 represents the experimental TRV solubility in neat ethanol; the symbol
represents the experimental TRV solubility in neat ethanol; the symbol  represents the reported solubility values of TRV in neat ethanol taken from reference [27]; the symbol
represents the reported solubility values of TRV in neat ethanol taken from reference [27]; the symbol  represents the reported solubility values of TRV in neat ethanol taken from reference [1]; the symbol
represents the reported solubility values of TRV in neat ethanol taken from reference [1]; the symbol  represents the reported solubility values of TRV in neat ethanol taken from reference [2].
represents the reported solubility values of TRV in neat ethanol taken from reference [2].
 represents the experimental solubility of TRV in (A) neat methanol, (B) neat n-propanol, and (C) neat n-butanol; the symbol
represents the experimental solubility of TRV in (A) neat methanol, (B) neat n-propanol, and (C) neat n-butanol; the symbol  represents the reported solubility values of TRV in (A) neat methanol, (B) neat n-propanol, and (C) neat n-butanol taken from reference [27]; the symbol
represents the reported solubility values of TRV in (A) neat methanol, (B) neat n-propanol, and (C) neat n-butanol taken from reference [27]; the symbol  represents the reported solubility values of TRV in (A) neat methanol, (B) neat n-propanol, and (C) neat n-butanol taken from reference [2].
represents the reported solubility values of TRV in (A) neat methanol, (B) neat n-propanol, and (C) neat n-butanol taken from reference [2].



References
-
- Sun X., Shao Y., Yan W. Measurement and correlation of solubilities of trans-resveratrol in ethanol + water and acetone + water mixed solvents at different temperatures. J. Chem. Eng. Data. 2008;53:2562–2566. doi: 10.1021/je800410b. - DOI
-
- Ha E.S., Park H., Lee S.K., Sim W.Y., Jiong J.S., Kim M.S. Equilibrium solubility and modeling of trans-resveratrol in dichloromethane and primary alcohol solvent mixtures at different temperatures. J. Mol. Liq. 2020;311:113363. doi: 10.1016/j.molliq.2020.113363. - DOI
-
- Gulcin I. Antioxidant properties of resveratrol: A structure-activity insight. Innov. Food Sci. Emerg. Technol. 2010;11:210–218. doi: 10.1016/j.ifset.2009.07.002. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

