Advances in the Synthesis of Ring-Fused Benzimidazoles and Imidazobenzimidazoles
- PMID: 34064312
- PMCID: PMC8124402
- DOI: 10.3390/molecules26092684
Advances in the Synthesis of Ring-Fused Benzimidazoles and Imidazobenzimidazoles
Abstract
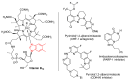
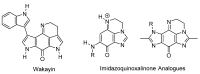
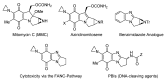
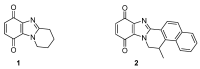
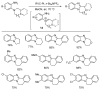
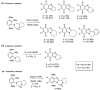
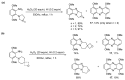
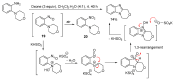
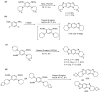
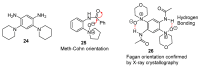
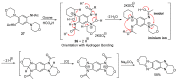
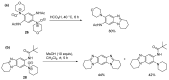
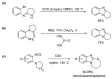
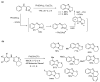
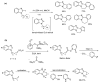
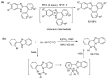
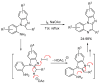
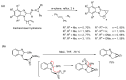
This review article provides a perspective on the synthesis of alicyclic and heterocyclic ring-fused benzimidazoles, imidazo[4,5-f]benzimidazoles, and imidazo[5,4-f]benzimidazoles. These heterocycles have a plethora of biological activities with the iminoquinone and quinone derivatives displaying potent bioreductive antitumor activity. Synthesis is categorized according to the cyclization reaction and mechanisms are detailed. Nitrobenzene reduction, cyclization of aryl amidines, lactams and isothiocyanates are described. Protocols include condensation, cross-dehydrogenative coupling with transition metal catalysis, annulation onto benzimidazole, often using CuI-catalysis, and radical cyclization with homolytic aromatic substitution. Many oxidative transformations are under metal-free conditions, including using thermal, photochemical, and electrochemical methods. Syntheses of diazole analogues of mitomycin C derivatives are described. Traditional oxidations of o-(cycloamino)anilines using peroxides in acid via the t-amino effect remain popular.
Keywords: green chemistry; halogen; heterocycle; hydrogen peroxide; imidazole; iodine; nitrosobenzene; oxone; palladium; quinone.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




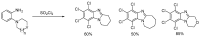
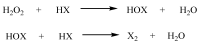





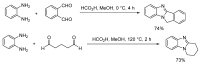
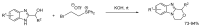

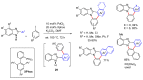
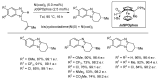






References
-
- Brink N.G., Folkers K. Vitamin B12 VI. 5,6-Dimethylbenzimidazole, a. degradation product of vitamin B12. J. Am. Chem. Soc. 1949;71:2951. doi: 10.1021/ja01176a532. - DOI
-
- Floyd J.C., Holliday E.R., Petrow V. Letters to the Editor. J. Pharm. Pharmacol. 1949;1:734–735. doi: 10.1111/j.2042-7158.1949.tb12490.x. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

