In Situ Maturated Early-Stage Human-Induced Pluripotent Stem Cell-Derived Cardiomyocytes Improve Cardiac Function by Enhancing Segmental Contraction in Infarcted Rats
- PMID: 34064343
- PMCID: PMC8147857
- DOI: 10.3390/jpm11050374
In Situ Maturated Early-Stage Human-Induced Pluripotent Stem Cell-Derived Cardiomyocytes Improve Cardiac Function by Enhancing Segmental Contraction in Infarcted Rats
Abstract
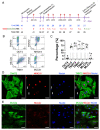
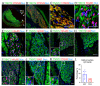
The scant ability of cardiomyocytes to proliferate makes heart regeneration one of the biggest challenges of science. Current therapies do not contemplate heart re-muscularization. In this scenario, stem cell-based approaches have been proposed to overcome this lack of regeneration. We hypothesize that early-stage hiPSC-derived cardiomyocytes (hiPSC-CMs) could enhance the cardiac function of rats after myocardial infarction (MI). Animals were subjected to the permanent occlusion of the left ventricle (LV) anterior descending coronary artery (LAD). Seven days after MI, early-stage hiPSC-CMs were injected intramyocardially. Rats were subjected to echocardiography pre-and post-treatment. Thirty days after the injections were administered, treated rats displayed 6.2% human cardiac grafts, which were characterized molecularly. Left ventricle ejection fraction (LVEF) was improved by 7.8% in cell-injected rats, while placebo controls showed an 18.2% deterioration. Additionally, cell-treated rats displayed a 92% and 56% increase in radial and circumferential strains, respectively. Human cardiac grafts maturate in situ, preserving proliferation with 10% Ki67 and 3% PHH3 positive nuclei. Grafts were perfused by host vasculature with no evidence for immune rejection nor ectopic tissue formations. Our findings support the use of early-stage hiPSC-CMs as an alternative therapy to treat MI. The next steps of preclinical development include efficacy studies in large animals on the path to clinical-grade regenerative therapy targeting human patients.
Keywords: cardiac function; cardiomyocytes; heart failure; human induced pluripotent stem cells; myocardial infarction; regeneration; stem cell-therapy.
Conflict of interest statement
The authors declared the following potential conflicts of interest concerning the research, authorship, and/or publication of this article: The authors D.B., E.T.F., J.C.C.O., M.V.N., A.F.R.Jr., S.R., I.O., R.V., M.V., E.C., and R.D were employees of PluriCell Biotech during the period in which the study was conducted. D.B., M.V., E.C., and R.D. own shares in PluriCell Biotech (D.B. and M.V. are co-founders). The other authors report no conflicts.
Figures




References
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

