Role of Transportin-SR2 in HIV-1 Nuclear Import
- PMID: 34064404
- PMCID: PMC8147801
- DOI: 10.3390/v13050829
Role of Transportin-SR2 in HIV-1 Nuclear Import
Abstract
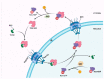
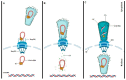
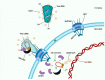
The HIV replication cycle depends on the interaction of viral proteins with proteins of the host. Unraveling host-pathogen interactions during the infection is of great importance for understanding the pathogenesis and the development of antiviral therapies. To date HIV uncoating and nuclear import are the most debated steps of the HIV-1 replication cycle. Despite numerous studies during past decades, there is still much controversy with respect to the identity and the role of viral and host factors involved in these processes. In this review, we provide a comprehensive overview on the role of transportin-SR2 as a host cell factor during active nuclear transport.
Keywords: CPSF6; HIV-1; TNPO3; TRN-SR2; capsid; integrase; nuclear import; transportin-SR2.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
CRISPR/Cas9-Induced Mutagenesis Corroborates the Role of Transportin-SR2 in HIV-1 Nuclear Import.Microbiol Spectr. 2021 Oct 31;9(2):e0133621. doi: 10.1128/Spectrum.01336-21. Epub 2021 Oct 6. Microbiol Spectr. 2021. PMID: 34612665 Free PMC article.
-
Inhibitors of the integrase-transportin-SR2 interaction block HIV nuclear import.Retrovirology. 2018 Jan 12;15(1):5. doi: 10.1186/s12977-018-0389-2. Retrovirology. 2018. PMID: 29329553 Free PMC article.
-
The HIV-1 integrase mutant R263A/K264A is 2-fold defective for TRN-SR2 binding and viral nuclear import.J Biol Chem. 2014 Sep 5;289(36):25351-61. doi: 10.1074/jbc.M113.533281. Epub 2014 Jul 25. J Biol Chem. 2014. PMID: 25063804 Free PMC article.
-
A model for cofactor use during HIV-1 reverse transcription and nuclear entry.Curr Opin Virol. 2014 Feb;4(100):32-6. doi: 10.1016/j.coviro.2013.11.003. Epub 2014 Jan 14. Curr Opin Virol. 2014. PMID: 24525292 Free PMC article. Review.
-
HIV Capsid and Integration Targeting.Viruses. 2021 Jan 18;13(1):125. doi: 10.3390/v13010125. Viruses. 2021. PMID: 33477441 Free PMC article. Review.
Cited by
-
The roles of CPSF6 in proliferation, apoptosis and tumorigenicity of lung adenocarcinoma.Aging (Albany NY). 2022 Nov 29;14(22):9300-9316. doi: 10.18632/aging.204407. Epub 2022 Nov 29. Aging (Albany NY). 2022. PMID: 36446361 Free PMC article.
-
The C-Terminal Domain of HIV-1 Integrase: A Swiss Army Knife for the Virus?Viruses. 2022 Jun 27;14(7):1397. doi: 10.3390/v14071397. Viruses. 2022. PMID: 35891378 Free PMC article. Review.
-
HIV-1 Capsid Rapidly Induces Long-Lived CPSF6 Puncta in Non-Dividing Cells, but Similar Puncta Already Exist in Uninfected T-Cells.Viruses. 2024 Apr 25;16(5):670. doi: 10.3390/v16050670. Viruses. 2024. PMID: 38793552 Free PMC article.
-
Complex Relationships between HIV-1 Integrase and Its Cellular Partners.Int J Mol Sci. 2022 Oct 15;23(20):12341. doi: 10.3390/ijms232012341. Int J Mol Sci. 2022. PMID: 36293197 Free PMC article. Review.
-
Tough Way In, Tough Way Out: The Complex Interplay of Host and Viral Factors in Nucleocytoplasmic Trafficking during HIV-1 Infection.Viruses. 2022 Nov 12;14(11):2503. doi: 10.3390/v14112503. Viruses. 2022. PMID: 36423112 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

