Immuno-Electron and Confocal Laser Scanning Microscopy of the Glycocalyx
- PMID: 34064459
- PMCID: PMC8147923
- DOI: 10.3390/biology10050402
Immuno-Electron and Confocal Laser Scanning Microscopy of the Glycocalyx
Abstract
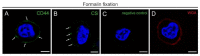
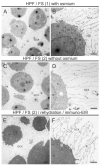
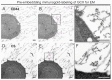
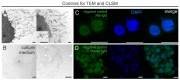
The glycocalyx (GCX), a pericellular carbohydrate rich hydrogel, forms a selective barrier that shields the cellular membrane, provides mechanical support, and regulates the transport and diffusion of molecules. The GCX is a fragile structure, making it difficult to study by transmission electron microscopy (TEM) and confocal laser scanning microscopy (CLSM). Sample preparation by conventional chemical fixation destroys the GCX, giving a false impression of its organization. An additional challenge is to process the GCX in a way that preserves its morphology and enhanced antigenicity to study its cell-specific composition. The aim of this study was to provide a protocol to preserve both antigen accessibility and the unique morphology of the GCX. We established a combined high pressure freezing (HPF), osmium-free freeze substitution (FS), rehydration, and pre-embedding immunogold labeling method for TEM. Our results showed specific immunogold labeling of GCX components expressed in human monocytic THP-1 cells, hyaluronic acid receptor (CD44) and chondroitin sulfate (CS), and maintained a well-preserved GCX morphology. We adapted the protocol for antigen localization by CLSM and confirmed the specific distribution pattern of GCX components. The presented combination of HPF, FS, rehydration, and immunolabeling for both TEM and CLSM offers the possibility for analyzing the morphology and composition of the unique GCX structure.
Keywords: confocal laser scanning microscopy; freeze-substitution; glycocalyx; high-pressure freezing; immuno-electron microscopy; pre-embedding immunogold labeling.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Constantinou P.E., Morgado M., Carson D.D. Transmembrane mucin expression and function in embryo implantation and placentation. Adv. Anat. Embryol. Cell Biol. 2015;216:51–68. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

