Prevalence, Virulence Gene Distribution and Alarming the Multidrug Resistance of Aeromonas hydrophila Associated with Disease Outbreaks in Freshwater Aquaculture
- PMID: 34064504
- PMCID: PMC8147934
- DOI: 10.3390/antibiotics10050532
Prevalence, Virulence Gene Distribution and Alarming the Multidrug Resistance of Aeromonas hydrophila Associated with Disease Outbreaks in Freshwater Aquaculture
Abstract
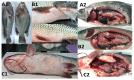
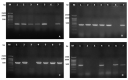
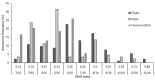
The study aims to evaluate the infection prevalence, virulence gene distribution and antimicrobial resistance of Aeromonas hydrophila associated in diseased outbreaks of cultured freshwater fish in Northern Vietnam. The confirmed A. hydrophila were screened for the presence of the five pitutative-virulence genes including aerolysin (aerA), hemolysin (hlyA), cytotonic enterotoxin (act), heat-labile cytotonic enterotoxin (alt), and heat-stable enterotoxin (ast), and examined the susceptibility to 16 antibiotics. A total of 236 A. hydrophila isolates were recovered and confirmed from 506 diseased fish by phenotypic tests, PCR assays, and gyrB, rpoB sequenced analyses, corresponding to the infection prevalence at 46.4%. A total of 88.9% of A. hydrophila isolates harbored at least one of the tested virulence genes. The genes aerA and act were most frequently found (80.5% and 80.1%, respectively) while the ast gene was absent in all isolates. The resistance to oxacillin, amoxicillin and vancomycin exhibited the highest frequencies (>70%), followed by erythromycin, oxytetracycline, florfenicol, and sulfamethoxazole/trimethoprim (9.3-47.2%). The multiple antibiotic resistance (MAR) index ranged between 0.13-0.88 with 74.7% of the isolates having MAR values higher than 0.2. The results present a warning for aquaculture farmers and managers in preventing the spread of A. hydrophila and minimizing antibiotic resistance of this pathogen in fish farming systems.
Keywords: Aeromonas hydrophila; Vietnam; antimicrobial resistances; freshwater fish; infection prevalence; virulence genes.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures




Similar articles
-
Virulence genes contributing to Aeromonas hydrophila pathogenicity in Oreochromis niloticus.Int Microbiol. 2019 Dec;22(4):479-490. doi: 10.1007/s10123-019-00075-3. Epub 2019 Apr 15. Int Microbiol. 2019. PMID: 30989358
-
Virulent and Multiple Antimicrobial Resistance Aeromonas hydrophila Isolated from Diseased Nile Tilapia Fish (Oreochromis niloticus) in Egypt with Sequencing of Some Virulence-Associated Genes.Biocontrol Sci. 2021;26(3):167-176. doi: 10.4265/bio.26.167. Biocontrol Sci. 2021. PMID: 34556619
-
Phenotypic, molecular detection, and Antibiotic Resistance Profile (MDR and XDR) of Aeromonas hydrophila isolated from Farmed Tilapia zillii and Mugil cephalus.BMC Vet Res. 2024 Mar 8;20(1):84. doi: 10.1186/s12917-024-03942-y. BMC Vet Res. 2024. PMID: 38459543 Free PMC article.
-
Detection of three virulence genes alt, ahp and aerA in Aeromonas hydrophila and their relationship with actual virulence to zebrafish.J Appl Microbiol. 2011 Mar;110(3):823-30. doi: 10.1111/j.1365-2672.2011.04944.x. Epub 2011 Feb 1. J Appl Microbiol. 2011. PMID: 21219556
-
A review on pathogenicity of Aeromonas hydrophila and their mitigation through medicinal herbs in aquaculture.Heliyon. 2023 Feb 28;9(3):e14088. doi: 10.1016/j.heliyon.2023.e14088. eCollection 2023 Mar. Heliyon. 2023. PMID: 36938468 Free PMC article. Review.
Cited by
-
Characterization and Genome Analyses of the Novel Phages P2 and vB_AhydM-H1 Targeting Aeromonas hydrophila.Phage (New Rochelle). 2024 Sep 16;5(3):162-172. doi: 10.1089/phage.2024.0014. eCollection 2024 Sep. Phage (New Rochelle). 2024. PMID: 39372357
-
Aerolysin gene characterization and antimicrobial resistance profile of Aeromonas hydrophila isolated from milkfish (Chanos chanos) in Gresik, Indonesia.Vet World. 2022 Jul;15(7):1759-1764. doi: 10.14202/vetworld.2022.1759-1764. Epub 2022 Jul 23. Vet World. 2022. PMID: 36185507 Free PMC article.
-
Synergistic Effect of Quercetin on Antibacterial Activity of Florfenicol Against Aeromonas hydrophila In Vitro and In Vivo.Antibiotics (Basel). 2022 Jul 10;11(7):929. doi: 10.3390/antibiotics11070929. Antibiotics (Basel). 2022. PMID: 35884183 Free PMC article.
-
Biocontrol of multidrug resistant pathogens isolated from fish farms using silver nanoparticles combined with hydrogen peroxide insight to its modulatory effect.Sci Rep. 2024 Apr 4;14(1):7971. doi: 10.1038/s41598-024-58349-4. Sci Rep. 2024. PMID: 38575637 Free PMC article.
-
Genomic characterization of Aeromonas spp. isolates from striped catfish with motile Aeromonas septicemia and human bloodstream infections in Vietnam.Microb Genom. 2024 May;10(5):001248. doi: 10.1099/mgen.0.001248. Microb Genom. 2024. PMID: 38739115 Free PMC article.
References
-
- Davis D., Nguyen T., Li M., Gatlin D.M., O’Keefe T. Advances in aquaculture nutrition: Catfish, tilapia and carp nutrition. In: Burnell G., Allan G., editors. New Technologies in Aquaculture. Woodhead Publishing; Cambridge, UK: 2009. pp. 440–458. - DOI
-
- Mzula A., Wambura P.N., Mdegela R.H., Shirima G.M. Present status of aquaculture and the challenge of bacterial diseases in freshwater farmed fish in Tanzania; A call for sustainable strategies. Aquac. Fish. 2020 doi: 10.1016/j.aaf.2020.05.003. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

