Multi-Smart and Scalable Bioligands-Free Nanomedical Platform for Intratumorally Targeted Tambjamine Delivery, a Difficult to Administrate Highly Cytotoxic Drug
- PMID: 34064518
- PMCID: PMC8147975
- DOI: 10.3390/biomedicines9050508
Multi-Smart and Scalable Bioligands-Free Nanomedical Platform for Intratumorally Targeted Tambjamine Delivery, a Difficult to Administrate Highly Cytotoxic Drug
Abstract
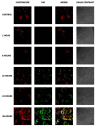
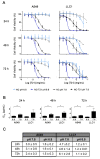
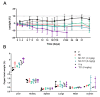
Cancer is one of the leading causes of mortality worldwide due, in part, to limited success of some current therapeutic approaches. The clinical potential of many promising drugs is restricted by their systemic toxicity and lack of selectivity towards cancer cells, leading to insufficient drug concentration at the tumor site. To overcome these hurdles, we developed a novel drug delivery system based on polyurea/polyurethane nanocapsules (NCs) showing pH-synchronized amphoteric properties that facilitate their accumulation and selectivity into acidic tissues, such as tumor microenvironment. We have demonstrated that the anticancer drug used in this study, a hydrophobic anionophore named T21, increases its cytotoxic activity in acidic conditions when nanoencapsulated, which correlates with a more efficient cellular internalization. A biodistribution assay performed in mice has shown that the NCs are able to reach the tumor and the observed systemic toxicity of the free drug is significantly reduced in vivo when nanoencapsulated. Additionally, T21 antitumor activity is preserved, accompanied by tumor mass reduction compared to control mice. Altogether, this work shows these NCs as a potential drug delivery system able to reach the tumor microenvironment, reducing the undesired systemic toxic effects. Moreover, these nanosystems are prepared under scalable methodologies and straightforward process, and provide tumor selectivity through a smart mechanism independent of targeting ligands.
Keywords: amphoteric nanocapsules; lung cancer treatment; pH-tunable; polymer nanocapsules; targeted drug delivery systems; tumor microenvironment.
Conflict of interest statement
Josep Rocas is the CEO of Ecopol Tech S.L. (were the nanocapsules were synthesized) and owns the patent WO2014114838A2. The rest of the authors declare no conflict of interest.
Figures









Similar articles
-
Novel Tumor-Targeted Self-Nanostructured and Compartmentalized Water-in-Oil-in-Water Polyurethane-Polyurea Nanocapsules for Cancer Theragnosis.Pharmaceutics. 2022 Dec 24;15(1):58. doi: 10.3390/pharmaceutics15010058. Pharmaceutics. 2022. PMID: 36678687 Free PMC article.
-
pH-Responsive Core-Shell Structured Nanoparticles for Triple-Stage Targeted Delivery of Doxorubicin to Tumors.ACS Appl Mater Interfaces. 2016 Sep 14;8(36):23498-508. doi: 10.1021/acsami.6b07173. Epub 2016 Aug 31. ACS Appl Mater Interfaces. 2016. PMID: 27558413
-
Stepwise targeted drug delivery to liver cancer cells for enhanced therapeutic efficacy by galactose-grafted, ultra-pH-sensitive micelles.Acta Biomater. 2017 Mar 15;51:363-373. doi: 10.1016/j.actbio.2017.01.031. Epub 2017 Jan 11. Acta Biomater. 2017. PMID: 28087485
-
Tumor-Acidity-Cleavable Maleic Acid Amide (TACMAA): A Powerful Tool for Designing Smart Nanoparticles To Overcome Delivery Barriers in Cancer Nanomedicine.Acc Chem Res. 2018 Nov 20;51(11):2848-2856. doi: 10.1021/acs.accounts.8b00195. Epub 2018 Oct 15. Acc Chem Res. 2018. PMID: 30346728 Review.
-
Targeted cancer therapy: conferring specificity to cytotoxic drugs.Acc Chem Res. 2008 Jan;41(1):98-107. doi: 10.1021/ar700108g. Epub 2007 Aug 18. Acc Chem Res. 2008. PMID: 17705444 Review.
Cited by
-
Novel Tumor-Targeted Self-Nanostructured and Compartmentalized Water-in-Oil-in-Water Polyurethane-Polyurea Nanocapsules for Cancer Theragnosis.Pharmaceutics. 2022 Dec 24;15(1):58. doi: 10.3390/pharmaceutics15010058. Pharmaceutics. 2022. PMID: 36678687 Free PMC article.
-
Synthesis of Degradable Polysulfamides via Sulfur(VI) Fluoride Exchange Click Polymerization of AB-Type Monomers.ACS Polym Au. 2023 Jan 17;3(3):259-266. doi: 10.1021/acspolymersau.2c00060. eCollection 2023 Jun 14. ACS Polym Au. 2023. PMID: 37334193 Free PMC article.
-
Editorial to the Special Issue "Theranostic Drug Delivery: Prospects and Problems".Biomedicines. 2024 Jul 10;12(7):1533. doi: 10.3390/biomedicines12071533. Biomedicines. 2024. PMID: 39062106 Free PMC article.
-
Improving Photodynamic Therapy Anticancer Activity of a Mitochondria-Targeted Coumarin Photosensitizer Using a Polyurethane-Polyurea Hybrid Nanocarrier.Biomacromolecules. 2022 Jul 11;23(7):2900-2913. doi: 10.1021/acs.biomac.2c00361. Epub 2022 Jun 13. Biomacromolecules. 2022. PMID: 35695426 Free PMC article.
-
Redox-responsive polyurethane-polyurea nanoparticles targeting to aortic endothelium and atherosclerosis.iScience. 2022 Oct 18;25(11):105390. doi: 10.1016/j.isci.2022.105390. eCollection 2022 Nov 18. iScience. 2022. PMID: 36345337 Free PMC article.
References
-
- Huang K., Ma H., Liu J., Huo S., Kumar A., Wei T., Zhang X., Jin S., Gan Y., Wang P.C., et al. Size-dependent localization and penetration of ultrasmall gold nanoparticles in cancer cells, multicellular spheroids, and tumors in vivo. ACS Nano. 2012;6:4483–4493. doi: 10.1021/nn301282m. - DOI - PMC - PubMed
Grants and funding
- BU092U16/Consejería de Educación de la Junta de Castilla y León
- BU067P20/Consejería de Educación de la Junta de Castilla y León
- PI18/00441/Instituto de Salud Carlos III
- EMC/2755/2017/ACCIÓ (Agengy for business copetitiveness, Generalitat de Catalunya)
- 2013 DI 028/Pla de doctorats Industrials de la Secretaria d'Universitats i Recerca del Departament d'Empresa i coneixement de la Generalitat de Catalunya

