ABA Metabolism and Homeostasis in Seed Dormancy and Germination
- PMID: 34064729
- PMCID: PMC8151144
- DOI: 10.3390/ijms22105069
ABA Metabolism and Homeostasis in Seed Dormancy and Germination
Abstract
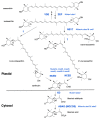
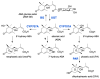
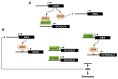
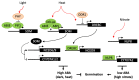
Abscisic acid (ABA) is a key hormone that promotes dormancy during seed development on the mother plant and after seed dispersal participates in the control of dormancy release and germination in response to environmental signals. The modulation of ABA endogenous levels is largely achieved by fine-tuning, in the different seed tissues, hormone synthesis by cleavage of carotenoid precursors and inactivation by 8'-hydroxylation. In this review, we provide an overview of the current knowledge on ABA metabolism in developing and germinating seeds; notably, how environmental signals such as light, temperature and nitrate control seed dormancy through the adjustment of hormone levels. A number of regulatory factors have been recently identified which functional relationships with major transcription factors, such as ABA INSENSITIVE3 (ABI3), ABI4 and ABI5, have an essential role in the control of seed ABA levels. The increasing importance of epigenetic mechanisms in the regulation of ABA metabolism gene expression is also described. In the last section, we give an overview of natural variations of ABA metabolism genes and their effects on seed germination, which could be useful both in future studies to better understand the regulation of ABA metabolism and to identify candidates as breeding materials for improving germination properties.
Keywords: abscisic acid; biosynthesis; catabolism; dormancy; germination; natural variation; seed; transcription factor.
Conflict of interest statement
The authors declare that there is no conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

