Rise of Clinical Studies in the Field of Machine Learning: A Review of Data Registered in ClinicalTrials.gov
- PMID: 34064827
- PMCID: PMC8151906
- DOI: 10.3390/ijerph18105072
Rise of Clinical Studies in the Field of Machine Learning: A Review of Data Registered in ClinicalTrials.gov
Abstract
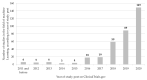
Although advances in machine-learning healthcare applications promise great potential for innovative medical care, few data are available on the translational status of these new technologies. We aimed to provide a comprehensive characterization of the development and status quo of clinical studies in the field of machine learning. For this purpose, we performed a registry-based analysis of machine-learning-related studies that were published and first available in the ClinicalTrials.gov database until 2020, using the database's study classification. In total, n = 358 eligible studies could be included in the analysis. Of these, 82% were initiated by academic institutions/university (hospitals) and 18% by industry sponsors. A total of 96% were national and 4% international. About half of the studies (47%) had at least one recruiting location in a country in North America, followed by Europe (37%) and Asia (15%). Most of the studies reported were initiated in the medical field of imaging (12%), followed by cardiology, psychiatry, anesthesia/intensive care medicine (all 11%) and neurology (10%). Although the majority of the clinical studies were still initiated in an academic research context, the first industry-financed projects on machine-learning-based algorithms are becoming visible. The number of clinical studies with machine-learning-related applications and the variety of medical challenges addressed serve to indicate their increasing importance in future clinical care. Finally, they also set a time frame for the adjustment of medical device-related regulation and governance.
Keywords: ClinicalTrials.gov; device regulation; digital health; machine learning; registry analysis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Grant J., Green L., Mason B. Basic research and health: A reassessment of the scientific basis for the support of biomedical science. Res. Eval. 2003;12:217–224. doi: 10.3152/147154403781776618. - DOI
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

