Fusicoccin (FC)-Induced Rapid Growth, Proton Extrusion and Membrane Potential Changes in Maize (Zea mays L.) Coleoptile Cells: Comparison to Auxin Responses
- PMID: 34065110
- PMCID: PMC8125996
- DOI: 10.3390/ijms22095017
Fusicoccin (FC)-Induced Rapid Growth, Proton Extrusion and Membrane Potential Changes in Maize (Zea mays L.) Coleoptile Cells: Comparison to Auxin Responses
Abstract
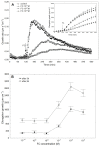
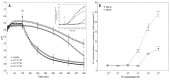
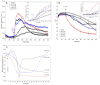
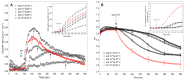
The fungal toxin fusicoccin (FC) induces rapid cell elongation, proton extrusion and plasma membrane hyperpolarization in maize coleoptile cells. Here, these three parameters were simultaneously measured using non-abraded and non-peeled segments with the incubation medium having access to their lumen. The dose-response curve for the FC-induced growth was sigmoidal shaped with the maximum at 10-6 M over 10 h. The amplitudes of the rapid growth and proton extrusion were significantly higher for FC than those for indole-3-acetic acid (IAA). The differences between the membrane potential changes that were observed in the presence of FC and IAA relate to the permanent membrane hyperpolarization for FC and transient hyperpolarization for IAA. It was also found that the lag times of the rapid growth, proton extrusion and membrane hyperpolarization were shorter for FC compared to IAA. At 30 °C, the biphasic kinetics of the IAA-induced growth rate could be changed into a monophasic (parabolic) one, which is characteristic for FC-induced rapid growth. It has been suggested that the rates of the initial phase of the FC- and IAA-induced growth involve two common mechanisms that consist of the proton pumps and potassium channels whose contribution to the action of both effectors on the rapid growth is different.
Keywords: Auxin (IAA); coleoptile segments; fusicoccin; growth; membrane potential; proton extrusion.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
The effect of H2O2 on elongation growth and oxidative stress in maize coleoptile cells treated with auxin and fusicoccin.Sci Rep. 2025 Apr 15;15(1):12903. doi: 10.1038/s41598-025-96912-9. Sci Rep. 2025. PMID: 40234506 Free PMC article.
-
Role of chloride ions in the promotion of auxin-induced growth of maize coleoptile segments.Ann Bot. 2014 Oct;114(5):1023-34. doi: 10.1093/aob/mcu170. Epub 2014 Aug 16. Ann Bot. 2014. PMID: 25129632 Free PMC article.
-
Some New Methodological and Conceptual Aspects of the "Acid Growth Theory" for the Auxin Action in Maize (Zea mays L.) Coleoptile Segments: Do Acid- and Auxin-Induced Rapid Growth Differ in Their Mechanisms?Int J Mol Sci. 2021 Feb 26;22(5):2317. doi: 10.3390/ijms22052317. Int J Mol Sci. 2021. PMID: 33652568 Free PMC article.
-
Fusicoccin counteracts the toxic effect of cadmium on the growth of maize coleoptile segments.Arch Environ Contam Toxicol. 2011 Nov;61(4):568-77. doi: 10.1007/s00244-011-9662-2. Epub 2011 Mar 19. Arch Environ Contam Toxicol. 2011. PMID: 21424219 Free PMC article.
-
Auxin biosynthesis in maize.Plant Biol (Stuttg). 2006 May;8(3):334-9. doi: 10.1055/s-2006-923883. Plant Biol (Stuttg). 2006. PMID: 16807825 Review.
Cited by
-
The Complexity of the Influence of Growth Substances, Heavy Metals, and Their Combination on the Volume Dynamics of Vacuoles Isolated from Red Beet (Beta vulgaris L.) Taproot Cells.Int J Mol Sci. 2024 Oct 9;25(19):10842. doi: 10.3390/ijms251910842. Int J Mol Sci. 2024. PMID: 39409172 Free PMC article.
-
The effect of H2O2 on elongation growth and oxidative stress in maize coleoptile cells treated with auxin and fusicoccin.Sci Rep. 2025 Apr 15;15(1):12903. doi: 10.1038/s41598-025-96912-9. Sci Rep. 2025. PMID: 40234506 Free PMC article.
-
The effect of DC electric field on the elongation growth, proton extrusion and membrane potential of Zea mays L. coleoptile cells; a laboratory study.BMC Plant Biol. 2022 Aug 3;22(1):389. doi: 10.1186/s12870-022-03778-4. BMC Plant Biol. 2022. PMID: 35922781 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

