Excessive Iron Induces Oxidative Stress Promoting Cellular Perturbations and Insulin Secretory Dysfunction in MIN6 Beta Cells
- PMID: 34065122
- PMCID: PMC8151797
- DOI: 10.3390/cells10051141
Excessive Iron Induces Oxidative Stress Promoting Cellular Perturbations and Insulin Secretory Dysfunction in MIN6 Beta Cells
Abstract
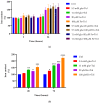
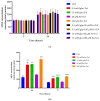
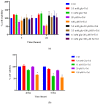
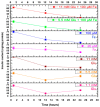
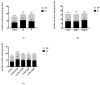
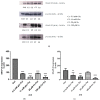
Exposure to high levels of glucose and iron are co-related to reactive oxygen species (ROS) generation and dysregulation of insulin synthesis and secretion, although the precise mechanisms are not well clarified. The focus of this study was to examine the consequences of exposure to high iron levels on MIN6 β-cells. MIN6 pseudoislets were exposed to 20 µM (control) or 100 µM (high) iron at predefined glucose levels (5.5 mM and 11 mM) at various time points (3, 24, 48, and 72 h). Total iron content was estimated by a colourimetric FerroZine™ assay in presence or absence of transferrin-bound iron. Cell viability was assessed by a resazurin dye-based assay, and ROS-mediated cellular oxidative stress was assessed by estimating malondialdehyde levels. β-cell iron absorption was determined by a ferritin immunoassay. Cellular insulin release and content was measured by an insulin immunoassay. Expression of SNAP-25, a key protein in the core SNARE complex that modulates vesicle exocytosis, was measured by immunoblotting. Our results demonstrate that exposure to high iron levels resulted in a 15-fold (48 h) and 4-fold (72 h) increase in cellular iron accumulation. These observations were consistent with data from oxidative stress analysis which demonstrated 2.7-fold higher levels of lipid peroxidation. Furthermore, exposure to supraphysiological (11 mM) levels of glucose and high iron (100 µM) at 72 h exerted the most detrimental effect on the MIN6 β-cell viability. The effect of high iron exposure on total cellular iron content was identical in the presence or absence of transferrin. High iron exposure (100 µM) resulted in a decrease of MIN6 insulin secretion (64% reduction) as well as cellular insulin content (10% reduction). Finally, a significant reduction in MIN6 β-cell SNAP-25 protein expression was evident at 48 h upon exposure to 100 µM iron. Our data suggest that exposure to high iron and glucose concentrations results in cellular oxidative damage and may initiate insulin secretory dysfunction in pancreatic β-cells by modulation of the exocytotic machinery.
Keywords: excess iron; impaired insulin secretion; oxidative stress; type 2 diabetes mellitus; β-cell.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Pioglitazone attenuates fatty acid-induced oxidative stress and apoptosis in pancreatic beta-cells.Diabetes Obes Metab. 2008 Jul;10(7):564-73. doi: 10.1111/j.1463-1326.2007.00749.x. Epub 2007 Jun 26. Diabetes Obes Metab. 2008. PMID: 17593232
-
Phenolic Acids Rescue Iron-Induced Damage in Murine Pancreatic Cells and Tissues.Molecules. 2023 May 14;28(10):4084. doi: 10.3390/molecules28104084. Molecules. 2023. PMID: 37241825 Free PMC article.
-
CFTR silencing in pancreatic β-cells reveals a functional impact on glucose-stimulated insulin secretion and oxidative stress response.Am J Physiol Endocrinol Metab. 2016 Feb 1;310(3):E200-12. doi: 10.1152/ajpendo.00333.2015. Epub 2015 Dec 1. Am J Physiol Endocrinol Metab. 2016. PMID: 26625901
-
Molecular mechanisms of ROS production and oxidative stress in diabetes.Biochem J. 2016 Dec 15;473(24):4527-4550. doi: 10.1042/BCJ20160503C. Biochem J. 2016. PMID: 27941030 Review.
-
Redox Status as a Key Driver of Healthy Pancreatic Beta-Cells.Physiol Res. 2024 Aug 30;73(S1):S139-S152. doi: 10.33549/physiolres.935259. Epub 2024 Apr 22. Physiol Res. 2024. PMID: 38647167 Free PMC article. Review.
Cited by
-
Radical oxygen species: an important breakthrough point for botanical drugs to regulate oxidative stress and treat the disorder of glycolipid metabolism.Front Pharmacol. 2023 May 12;14:1166178. doi: 10.3389/fphar.2023.1166178. eCollection 2023. Front Pharmacol. 2023. PMID: 37251336 Free PMC article. Review.
-
Correlations Between Iron Status and Body Composition in Patients With Type 2 Diabetes Mellitus.Front Nutr. 2022 Jul 13;9:911860. doi: 10.3389/fnut.2022.911860. eCollection 2022. Front Nutr. 2022. PMID: 35911095 Free PMC article.
-
RISING STARS: Evidence for established and emerging forms of β-cell death.J Endocrinol. 2024 Jul 4;262(2):e230378. doi: 10.1530/JOE-23-0378. Print 2024 Aug 1. J Endocrinol. 2024. PMID: 38842911 Free PMC article. Review.
-
Beneficial Contribution to Glucose Homeostasis by an Agro-Food Waste Product Rich in Abscisic Acid: Results from a Randomized Controlled Trial.Foods. 2022 Aug 31;11(17):2637. doi: 10.3390/foods11172637. Foods. 2022. PMID: 36076823 Free PMC article.
-
Targeting ferroptosis: opportunities and challenges of mesenchymal stem cell therapy for type 1 diabetes mellitus.Stem Cell Res Ther. 2025 Feb 4;16(1):47. doi: 10.1186/s13287-025-04188-7. Stem Cell Res Ther. 2025. PMID: 39901210 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

