A Negative Energy Balance Is Associated with Metabolic Dysfunctions in the Hypothalamus of a Humanized Preclinical Model of Alzheimer's Disease, the 5XFAD Mouse
- PMID: 34065168
- PMCID: PMC8161294
- DOI: 10.3390/ijms22105365
A Negative Energy Balance Is Associated with Metabolic Dysfunctions in the Hypothalamus of a Humanized Preclinical Model of Alzheimer's Disease, the 5XFAD Mouse
Abstract
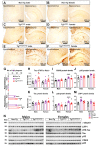
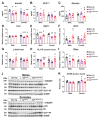
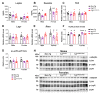
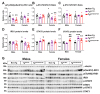
Increasing evidence links metabolic disorders with neurodegenerative processes including Alzheimer's disease (AD). Late AD is associated with amyloid (Aβ) plaque accumulation, neuroinflammation, and central insulin resistance. Here, a humanized AD model, the 5xFAD mouse model, was used to further explore food intake, energy expenditure, neuroinflammation, and neuroendocrine signaling in the hypothalamus. Experiments were performed on 6-month-old male and female full transgenic (Tg5xFAD/5xFAD), heterozygous (Tg5xFAD/-), and non-transgenic (Non-Tg) littermates. Although histological analysis showed absence of Aβ plaques in the hypothalamus of 5xFAD mice, this brain region displayed increased protein levels of GFAP and IBA1 in both Tg5xFAD/- and Tg5xFAD/5xFAD mice and increased expression of IL-1β in Tg5xFAD/5xFAD mice, suggesting neuroinflammation. This condition was accompanied by decreased body weight, food intake, and energy expenditure in both Tg5xFAD/- and Tg5xFAD/5xFAD mice. Negative energy balance was associated with altered circulating levels of insulin, GLP-1, GIP, ghrelin, and resistin; decreased insulin and leptin hypothalamic signaling; dysregulation in main metabolic sensors (phosphorylated IRS1, STAT5, AMPK, mTOR, ERK2); and neuropeptides controlling energy balance (NPY, AgRP, orexin, MCH). These results suggest that glial activation and metabolic dysfunctions in the hypothalamus of a mouse model of AD likely result in negative energy balance, which may contribute to AD pathogenesis development.
Keywords: 5xFAD; Alzheimer’s disease; energy expenditure; hypothalamus; insulin signaling; neuroinflammation.
Conflict of interest statement
C.S. declares he receives a salary from and has shares in the Euronutra company. The remaining authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures




Similar articles
-
Dietary administration of D-chiro-inositol attenuates sex-specific metabolic imbalances in the 5xFAD mouse model of Alzheimer's disease.Biomed Pharmacother. 2022 Jun;150:112994. doi: 10.1016/j.biopha.2022.112994. Epub 2022 Apr 26. Biomed Pharmacother. 2022. PMID: 35483188
-
Butyrylcholinesterase-knockout reduces fibrillar β-amyloid and conserves 18FDG retention in 5XFAD mouse model of Alzheimer's disease.Brain Res. 2017 Sep 15;1671:102-110. doi: 10.1016/j.brainres.2017.07.009. Epub 2017 Jul 17. Brain Res. 2017. PMID: 28729192
-
MK-0677, a Ghrelin Agonist, Alleviates Amyloid Beta-Related Pathology in 5XFAD Mice, an Animal Model of Alzheimer's Disease.Int J Mol Sci. 2018 Jun 18;19(6):1800. doi: 10.3390/ijms19061800. Int J Mol Sci. 2018. PMID: 29912176 Free PMC article.
-
Understanding the Amyloid Hypothesis in Alzheimer's Disease.J Alzheimers Dis. 2019;68(2):493-510. doi: 10.3233/JAD-180802. J Alzheimers Dis. 2019. PMID: 30883346 Review.
-
Behaviour Hallmarks in Alzheimer's Disease 5xFAD Mouse Model.Int J Mol Sci. 2024 Jun 20;25(12):6766. doi: 10.3390/ijms25126766. Int J Mol Sci. 2024. PMID: 38928472 Free PMC article. Review.
Cited by
-
The Emerging Role of Metabolism in Brain-Heart Axis: New Challenge for the Therapy and Prevention of Alzheimer Disease. May Thioredoxin Interacting Protein (TXNIP) Play a Role?Biomolecules. 2021 Nov 8;11(11):1652. doi: 10.3390/biom11111652. Biomolecules. 2021. PMID: 34827650 Free PMC article. Review.
-
Hypothalamic Regulation of Obesity.Int J Mol Sci. 2021 Dec 15;22(24):13459. doi: 10.3390/ijms222413459. Int J Mol Sci. 2021. PMID: 34948254 Free PMC article.
-
Amyloid β Proteoforms Elucidated by Quantitative LC/MS in the 5xFAD Mouse Model of Alzheimer's Disease.J Proteome Res. 2023 Nov 3;22(11):3475-3488. doi: 10.1021/acs.jproteome.3c00353. Epub 2023 Oct 17. J Proteome Res. 2023. PMID: 37847596 Free PMC article.
-
Sex Differences in Hypothalamic Changes and the Metabolic Response of TgAPP Mice to a High Fat Diet.Front Neuroanat. 2022 Jul 26;16:910477. doi: 10.3389/fnana.2022.910477. eCollection 2022. Front Neuroanat. 2022. PMID: 35958733 Free PMC article.
-
Reduction of Neuroinflammation as a Common Mechanism of Action of Anorexigenic and Orexigenic Peptide Analogues in the Triple Transgenic Mouse Model of Alzheimer´s Disease.J Neuroimmune Pharmacol. 2025 Feb 11;20(1):18. doi: 10.1007/s11481-025-10174-w. J Neuroimmune Pharmacol. 2025. PMID: 39932627 Free PMC article.
References
-
- Talbot K., Wang H.Y., Kazi H., Han L.Y., Bakshi K.P., Stucky A., Fuino R.L., Kawaguchi K.R., Samoyedny A.J., Wilson R.S., et al. Demonstrated brain insulin resistance in Alzheimer’s disease patients is associated with IGF-1 resistance, IRS-1 dysregulation, and cognitive decline. J. Clin. Investig. 2012;122:1316–1338. doi: 10.1172/JCI59903. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
- EULACH16/T010131/H2020 European Research Council
- RTC-2016-4983-1/Ministerio de Economía, Industria y Competitividad, Gobierno de España
- PI19/01577/Instituto de Salud Carlos III
- PI19/00343/Instituto de Salud Carlos III
- PNSD 2019/040/Ministerio de Sanidad, Consumo y Bienestar Social
- PNSD 2020/048/Ministerio de Sanidad, Consumo y Bienestar Social
- P18-TP-5194/Consejería de Transformación Económica, Industria, Conocimiento y Universidades. Junta de Andalucía
- CPII17/00024/Instituto de Salud Carlos III
- CP19/00068/Instituto de Salud Carlos III
- FI20/00227/Instituto de Salud Carlos III
- IFI18/00042/Instituto de Salud Carlos III
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

