Tau Exon 10 Inclusion by PrPC through Downregulating GSK3β Activity
- PMID: 34065232
- PMCID: PMC8161268
- DOI: 10.3390/ijms22105370
Tau Exon 10 Inclusion by PrPC through Downregulating GSK3β Activity
Abstract
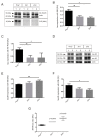
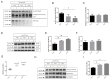
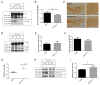
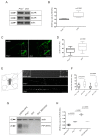
Tau protein is largely responsible for tauopathies, including Alzheimer's disease (AD), where it accumulates in the brain as insoluble aggregates. Tau mRNA is regulated by alternative splicing, and inclusion or exclusion of exon 10 gives rise to the 3R and 4R isoforms respectively, whose balance is physiologically regulated. In this sense, one of the several factors that regulate alternative splicing of tau is GSK3β, whose activity is inhibited by the cellular prion protein (PrPC), which has different physiological functions in neuroprotection and neuronal differentiation. Moreover, a relationship between PrPC and tau expression levels has been reported during AD evolution. For this reason, in this study we aimed to analyze the role of PrPC and the implication of GSK3β in the regulation of tau exon 10 alternative splicing. We used AD human samples and mouse models of PrPC ablation and tau overexpression. In addition, we used primary neuronal cultures to develop functional studies. Our results revealed a paralleled association between PrPC expression and tau 4R isoforms in all models analyzed. In this sense, reduction or ablation of PrPC levels induces an increase in tau 3R/4R balance. More relevantly, our data points to GSK3β activity downstream from PrPC in this phenomenon. Our results indicate that PrPC plays a role in tau exon 10 inclusion through the inhibitory capacity of GSK3β.
Keywords: Alzheimer’s disease; GSK3β; alternative splicing; cellular prion protein; microtubule-associated protein tau; tauopathies.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Lowe J., Kalaria R. Dementia. In: Love S., Budka H., Ironside J., Perry A., editors. Greenfield’s Neuropathology. 9th ed. CRC Press; Boca Raton, FL, USA: 2015. pp. 858–973.
MeSH terms
Substances
Grants and funding
- RTI2018-099773-B-I00/Spanish Ministry of Science, Innovation and Universities
- FPU15/02705/CERCA Programme, the Spanish Ministry of Education and Professional Formation
- SGR2017-648/Commission for Universities and Research of the Department of Innovation, Universities, and Enterprise of the Generalitat de Catalunya
- CMED2018-2/CIBERNED
- LCF/PR/HR19/52160007/"la Caixa" Foundation (ID 100010434)
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

