Activity of sEH and Oxidant Status during Systemic Bovine Coliform Mastitis
- PMID: 34065244
- PMCID: PMC8161397
- DOI: 10.3390/antiox10050812
Activity of sEH and Oxidant Status during Systemic Bovine Coliform Mastitis
Abstract
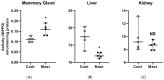
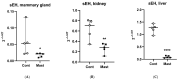
Bovine coliform mastitis presents treatment challenges because of systemic inflammation and oxidative stress. Soluble epoxide hydrolase (sEH) is a promising therapeutic target in conditions characterized by inflammation and oxidative stress but has not been evaluated in cattle. We compared sEH activity and oxidant status in healthy Holstein dairy cows to those with systemic coliform mastitis (n = 5/group) using complementary approaches. First, the activity of sEH on [3H]-trans-diphenyl-propene oxide (tDPPO) was assessed ex vivo using tissue homogenates (mammary, liver, and kidney). Second, the concentrations of sEH substrates and metabolites in plasma, milk, and urine were determined as an index of in vivo sEH activity. Oxidant status was assessed in serum and milk. Data were analyzed by non-parametric methods. Metabolism of tDPPO was greater in mammary tissues from cows with coliform mastitis compared to controls. In contrast, ratios of sEH substrates and metabolites predicted lower sEH activity in cows with coliform mastitis than controls. Milk oxidant status showed greater prooxidant levels in coliform mastitis cows. Cows with coliform mastitis exhibit increased sEH activity in mammary tissue; at the same time, milk oxidant status is increased. Future studies should characterize sEH activity and oxidant status patterns and explore therapies targeting sEH during coliform mastitis.
Keywords: cytochrome P450; inflammation; mastitis; oxidative stress; oxylipids; soluble epoxide hydrolase.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
15-F2t-Isoprostane Concentrations and Oxidant Status in Lactating Dairy Cattle with Acute Coliform Mastitis.J Vet Intern Med. 2016 Jan-Feb;30(1):339-47. doi: 10.1111/jvim.13793. Epub 2015 Nov 14. J Vet Intern Med. 2016. PMID: 26566597 Free PMC article.
-
Flunixin Meglumine Reduces Milk Isoprostane Concentrations in Holstein Dairy Cattle Suffering from Acute Coliform Mastitis.Antioxidants (Basel). 2021 May 24;10(6):834. doi: 10.3390/antiox10060834. Antioxidants (Basel). 2021. PMID: 34073753 Free PMC article.
-
Polyunsaturated fatty acids influence differential biosynthesis of oxylipids and other lipid mediators during bovine coliform mastitis.J Dairy Sci. 2015 Sep;98(9):6202-15. doi: 10.3168/jds.2015-9570. Epub 2015 Jul 8. J Dairy Sci. 2015. PMID: 26162796
-
The proteomic advantage: label-free quantification of proteins expressed in bovine milk during experimentally induced coliform mastitis.Vet Immunol Immunopathol. 2010 Dec 15;138(4):252-66. doi: 10.1016/j.vetimm.2010.10.004. Epub 2010 Oct 14. Vet Immunol Immunopathol. 2010. PMID: 21067814 Review.
-
Symposium review: Oxylipids and the regulation of bovine mammary inflammatory responses.J Dairy Sci. 2018 Jun;101(6):5629-5641. doi: 10.3168/jds.2017-13855. Epub 2018 Feb 4. J Dairy Sci. 2018. PMID: 29397182 Review.
Cited by
-
Sanguinarine Enhances the Integrity of the Blood-Milk Barrier and Inhibits Oxidative Stress in Lipopolysaccharide-Stimulated Mastitis.Cells. 2022 Nov 18;11(22):3658. doi: 10.3390/cells11223658. Cells. 2022. PMID: 36429086 Free PMC article.
-
Role of Selenium and Vitamins E and B9 in the Alleviation of Bovine Mastitis during the Periparturient Period.Antioxidants (Basel). 2022 Mar 29;11(4):657. doi: 10.3390/antiox11040657. Antioxidants (Basel). 2022. PMID: 35453342 Free PMC article. Review.
-
Effect of weaning strategies on biosynthesis of oxylipids in Holstein dairy calves.JDS Commun. 2024 Sep 18;6(1):149-153. doi: 10.3168/jdsc.2024-0600. eCollection 2025 Jan. JDS Commun. 2024. PMID: 39877181 Free PMC article.
-
Blood neutrophil extracellular traps: a novel target for the assessment of mammary health in transition dairy cows.J Anim Sci Biotechnol. 2022 Nov 16;13(1):131. doi: 10.1186/s40104-022-00782-4. J Anim Sci Biotechnol. 2022. PMID: 36380371 Free PMC article.
References
-
- Moroni P., Nydam D.V., Ospina P.A., Scillieri-Smith J.C., Virkler P.D., Watters R.D., Welcome F.L., Zurakowski M.J., Ducharme N.G., Yeager A.E. Diseases of the Teats and Udder. In: Peek S., Divers T.J., editors. Rebhun’s Diseases of Dairy Cattle. 3rd ed. Elsevier; St Louis, MO, USA: 2017. pp. 389–465.

