Improving Medication Regimen Recommendation for Parkinson's Disease Using Sensor Technology
- PMID: 34065245
- PMCID: PMC8160757
- DOI: 10.3390/s21103553
Improving Medication Regimen Recommendation for Parkinson's Disease Using Sensor Technology
Abstract
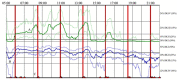
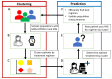
Parkinson's disease medication treatment planning is generally based on subjective data obtained through clinical, physician-patient interactions. The Personal KinetiGraph™ (PKG) and similar wearable sensors have shown promise in enabling objective, continuous remote health monitoring for Parkinson's patients. In this proof-of-concept study, we propose to use objective sensor data from the PKG and apply machine learning to cluster patients based on levodopa regimens and response. The resulting clusters are then used to enhance treatment planning by providing improved initial treatment estimates to supplement a physician's initial assessment. We apply k-means clustering to a dataset of within-subject Parkinson's medication changes-clinically assessed by the MDS-Unified Parkinson's Disease Rating Scale-III (MDS-UPDRS-III) and the PKG sensor for movement staging. A random forest classification model was then used to predict patients' cluster allocation based on their respective demographic information, MDS-UPDRS-III scores, and PKG time-series data. Clinically relevant clusters were partitioned by levodopa dose, medication administration frequency, and total levodopa equivalent daily dose-with the PKG providing similar symptomatic assessments to physician MDS-UPDRS-III scores. A random forest classifier trained on demographic information, MDS-UPDRS-III scores, and PKG time-series data was able to accurately classify subjects of the two most demographically similar clusters with an accuracy of 86.9%, an F1 score of 90.7%, and an AUC of 0.871. A model that relied solely on demographic information and PKG time-series data provided the next best performance with an accuracy of 83.8%, an F1 score of 88.5%, and an AUC of 0.831, hence further enabling fully remote assessments. These computational methods demonstrate the feasibility of using sensor-based data to cluster patients based on their medication responses with further potential to assist with medication recommendations.
Keywords: PKG; Parkinson’s disease; clustering; decision support tool; levodopa; machine learning; regimen; remote assessment; wearable sensors.
Conflict of interest statement
F.B.N. had no competing interests to declare at the time of the data collection, analysis, and initial publication. At present, F.B.N. is employed by Global Kinetics. He has no other competing interests. The other authors declare no conflicts of interest.
Figures


Similar articles
-
Cost-effectiveness analysis of the Parkinson's KinetiGraph and clinical assessment in the management of Parkinson's disease.J Med Econ. 2022 Jan-Dec;25(1):774-782. doi: 10.1080/13696998.2022.2080437. J Med Econ. 2022. PMID: 35593687
-
Test, track, treat using wearable sensors for management of Parkinson's disease: 12‑month prospective observational United Arab Emirates study using Parkinson's Kinetograph (EmPark-PKG Study).J Neural Transm (Vienna). 2025 Apr;132(4):591-601. doi: 10.1007/s00702-024-02873-0. Epub 2024 Dec 27. J Neural Transm (Vienna). 2025. PMID: 39730960 Free PMC article.
-
Treatment Detection and Movement Disorder Society-Unified Parkinson's Disease Rating Scale, Part III Estimation Using Finger Tapping Tasks.Mov Disord. 2023 Oct;38(10):1795-1805. doi: 10.1002/mds.29520. Epub 2023 Jul 4. Mov Disord. 2023. PMID: 37401265
-
Ropinirole: a review of its use in the management of Parkinson's disease.Drugs. 2000 Jul;60(1):115-37. doi: 10.2165/00003495-200060010-00007. Drugs. 2000. PMID: 10929932 Review.
-
Comparison of selegiline and levodopa combination therapy versus levodopa monotherapy in the treatment of Parkinson's disease: a meta-analysis.Aging Clin Exp Res. 2020 May;32(5):769-779. doi: 10.1007/s40520-019-01232-4. Epub 2019 Jun 7. Aging Clin Exp Res. 2020. PMID: 31175606 Review.
Cited by
-
Identification and quantitative assessment of motor complications in Parkinson's disease using the Parkinson's KinetiGraph™.Front Aging Neurosci. 2023 Aug 1;15:1142268. doi: 10.3389/fnagi.2023.1142268. eCollection 2023. Front Aging Neurosci. 2023. PMID: 37593376 Free PMC article.
-
Devices for remote continuous monitoring of people with Parkinson's disease: a systematic review and cost-effectiveness analysis.Health Technol Assess. 2024 Jul;28(30):1-187. doi: 10.3310/YDSL3294. Health Technol Assess. 2024. PMID: 39021200 Free PMC article.
-
A machine learning approach to predict quality of life changes in patients with Parkinson's Disease.Ann Clin Transl Neurol. 2023 Mar;10(3):312-320. doi: 10.1002/acn3.51577. Epub 2023 Feb 7. Ann Clin Transl Neurol. 2023. PMID: 36751867 Free PMC article.
-
Systematic review of wearables assessing medication effect on motor function and symptoms in Parkinson's disease.NPJ Parkinsons Dis. 2025 May 22;11(1):135. doi: 10.1038/s41531-025-00943-y. NPJ Parkinsons Dis. 2025. PMID: 40404728 Free PMC article.
-
Internet of Things Technologies and Machine Learning Methods for Parkinson's Disease Diagnosis, Monitoring and Management: A Systematic Review.Sensors (Basel). 2022 Feb 24;22(5):1799. doi: 10.3390/s22051799. Sensors (Basel). 2022. PMID: 35270944 Free PMC article.
References
-
- Feigin V.L., Abajobir A.A., Abate K.H., Abd-Allah F., Abdulle A.M., Abera S.F., Abyu G.Y., Ahmed M.B., Aichour A.N., Aichour I. Global, regional, and national burden of neurological disorders during 1990–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet Neurol. 2017;16:877–897. doi: 10.1016/S1474-4422(17)30299-5. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

