Simulations on Simple Models of Connexin Hemichannels Indicate That Ca2+ Blocking Is Not a Pure Electrostatic Effect
- PMID: 34065259
- PMCID: PMC8161212
- DOI: 10.3390/membranes11050372
Simulations on Simple Models of Connexin Hemichannels Indicate That Ca2+ Blocking Is Not a Pure Electrostatic Effect
Abstract
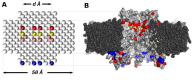
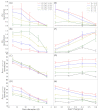
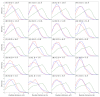
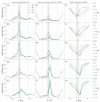
Connexin hemichannels allow the unspecific but regulated interchange of molecules from ions to second messenger and ATP, between the eukariotic cell and its extracellular space. The transport of ions and water through hemichannels is important for physiological functions and also in the progression of several pathological conditions. Extracellular Ca2+ concentration is one of the regulators that drives the channel to a closed state. However the relation between their functional and structural states is far for being totally understood. In this work, we modelled connexin hemichannels using simple systems based on a fixed array of carbon atoms and assess the Ca2+ regulation using molecular dynamics simulations. The two proposed mechanism described so far for calcium action were studied combined, e.g., an electrostatic effect and a pore stretching. Our results show that the addition of positive charge density inside the channel cannot stop the flow of potassium, chloride nor water. Only a pore stretching at the center of the pore can explain the channel blocking.
Keywords: calcium-binding; connexin; hemichannel; simulation.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures

Similar articles
-
Emerging issues of connexin channels: biophysics fills the gap.Q Rev Biophys. 2001 Aug;34(3):325-472. doi: 10.1017/s0033583501003705. Q Rev Biophys. 2001. PMID: 11838236 Review.
-
Mechanism of gating by calcium in connexin hemichannels.Proc Natl Acad Sci U S A. 2016 Dec 6;113(49):E7986-E7995. doi: 10.1073/pnas.1609378113. Epub 2016 Nov 21. Proc Natl Acad Sci U S A. 2016. PMID: 27872296 Free PMC article.
-
At the cross-point of connexins, calcium, and ATP: blocking hemichannels inhibits vasoconstriction of rat small mesenteric arteries.Cardiovasc Res. 2017 Feb;113(2):195-206. doi: 10.1093/cvr/cvw215. Epub 2016 Sep 27. Cardiovasc Res. 2017. PMID: 27677282
-
Insights on the mechanisms of Ca(2+) regulation of connexin26 hemichannels revealed by human pathogenic mutations (D50N/Y).J Gen Physiol. 2013 Jul;142(1):23-35. doi: 10.1085/jgp.201210893. J Gen Physiol. 2013. PMID: 23797420 Free PMC article.
-
Regulation of connexin hemichannel activity by membrane potential and the extracellular calcium in health and disease.Neuropharmacology. 2013 Dec;75:479-90. doi: 10.1016/j.neuropharm.2013.03.040. Epub 2013 Apr 12. Neuropharmacology. 2013. PMID: 23587648 Review.
Cited by
-
Calcium Regulation of Connexin Hemichannels.Int J Mol Sci. 2024 Jun 15;25(12):6594. doi: 10.3390/ijms25126594. Int J Mol Sci. 2024. PMID: 38928300 Free PMC article. Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

