Cripto-1 as a Potential Target of Cancer Stem Cells for Immunotherapy
- PMID: 34065315
- PMCID: PMC8160785
- DOI: 10.3390/cancers13102491
Cripto-1 as a Potential Target of Cancer Stem Cells for Immunotherapy
Abstract
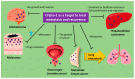
The immune system has been found to be suppressed in cancer patients. Cancer cells are extremely resistant to chemotherapeutic drugs, conventional immunotherapy, or cancer antigen vaccine therapy. Cancer immunotherapy, which is mainly based on immune checkpoint inhibitors, such as those for PD-1, PD-L1, and CTLA4, is an effective treatment method. However, no immunotherapeutic target has been found that retains validity in the face of tumor diversity. The transforming growth factor (TGF)-β cytokine family possesses broad biological activity and is involved in the induction and/or transdifferentiation of helper T cells, which are important in immunotherapy. Nodal is a member of the TGF-β family playing important roles in tissue stem cells and cancer stem cells (CSCs), interacting with the co-receptor Cripto-1, as well as with Activin type IB (Alk4) and Activin typeIIreceptors, and maintaining stemness and Notch and Wnt/β-catenin signaling in CSCs. In recent years, it has been reported that Cripto-1 could be a potential therapeutic target in CSCs. Here, we review the accumulated literature on the molecular mechanisms by which Cripto-1 functions in CSCs and discuss the potential of Cripto-1 as an immunotherapeutic target in CSCs.
Keywords: Cripto-1; TGF-β; antibody; cancer stem cells; immunotherapy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Cripto forms a complex with activin and type II activin receptors and can block activin signaling.Proc Natl Acad Sci U S A. 2003 Apr 29;100(9):5193-8. doi: 10.1073/pnas.0531290100. Epub 2003 Apr 7. Proc Natl Acad Sci U S A. 2003. PMID: 12682303 Free PMC article.
-
Antibody blockade of the Cripto CFC domain suppresses tumor cell growth in vivo.J Clin Invest. 2003 Aug;112(4):575-87. doi: 10.1172/JCI17788. J Clin Invest. 2003. PMID: 12925698 Free PMC article.
-
Cell-type specific regulation of myostatin signaling.FASEB J. 2012 Apr;26(4):1462-72. doi: 10.1096/fj.11-191189. Epub 2011 Dec 27. FASEB J. 2012. PMID: 22202673
-
An evolving web of signaling networks regulated by Cripto-1.Growth Factors. 2012 Feb;30(1):13-21. doi: 10.3109/08977194.2011.641962. Epub 2011 Dec 12. Growth Factors. 2012. PMID: 22149969 Review.
-
New Insights into Cancer Targeted Therapy: Nodal and Cripto-1 as Attractive Candidates.Int J Mol Sci. 2021 Jul 22;22(15):7838. doi: 10.3390/ijms22157838. Int J Mol Sci. 2021. PMID: 34360603 Free PMC article. Review.
Cited by
-
Induction, growth, drug resistance, and metastasis: A comprehensive summary of the relationship between STAT3 and gastric cancer.Heliyon. 2024 Sep 4;10(18):e37263. doi: 10.1016/j.heliyon.2024.e37263. eCollection 2024 Sep 30. Heliyon. 2024. PMID: 39309860 Free PMC article. Review.
-
Production in Bacteria and Characterization of Engineered Humanized Fab Fragment against the Nodal Protein.Pharmaceuticals (Basel). 2023 Aug 10;16(8):1130. doi: 10.3390/ph16081130. Pharmaceuticals (Basel). 2023. PMID: 37631045 Free PMC article.
-
CAR-Based Immunotherapy of Solid Tumours-A Survey of the Emerging Targets.Cancers (Basel). 2023 Feb 11;15(4):1171. doi: 10.3390/cancers15041171. Cancers (Basel). 2023. PMID: 36831514 Free PMC article. Review.
-
CRISPR/Cas9 as a therapeutic tool for triple negative breast cancer: from bench to clinics.Front Mol Biosci. 2023 Jul 4;10:1214489. doi: 10.3389/fmolb.2023.1214489. eCollection 2023. Front Mol Biosci. 2023. PMID: 37469704 Free PMC article. Review.
-
Whence CRIPTO: The Reemergence of an Oncofetal Factor in 'Wounds' That Fail to Heal.Int J Mol Sci. 2021 Sep 21;22(18):10164. doi: 10.3390/ijms221810164. Int J Mol Sci. 2021. PMID: 34576327 Free PMC article. Review.
References
-
- Kumagai S., Togashi Y., Kamada T., Sugiyama E., Nishinakamura H., Takeuchi Y., Vitaly K., Itahashi K., Maeda Y., Matsui S., et al. The PD-1 expression balance between effector and regulatory T cells predicts the clinical efficacy of PD-1 blockade therapies. Nat. Immunol. 2020;21:1346–1358. doi: 10.1038/s41590-020-0769-3. - DOI - PubMed
-
- Freeman G.J., Long A.J., Iwai Y., Bourque K., Chernova T., Nishimura H., Fitz L.J., Malenkovich N., Okazaki T., Byrne M.C., et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J. Exp. Med. 2000;192:1027–1034. doi: 10.1084/jem.192.7.1027. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

