Patterns of Recurrence after Neoadjuvant Therapy in Early Breast Cancer, according to the Residual Cancer Burden Index and Reductions in Neoadjuvant Treatment Intensity
- PMID: 34065332
- PMCID: PMC8161089
- DOI: 10.3390/cancers13102492
Patterns of Recurrence after Neoadjuvant Therapy in Early Breast Cancer, according to the Residual Cancer Burden Index and Reductions in Neoadjuvant Treatment Intensity
Abstract
Background: The prognostic performance of the residual cancer burden (RCB) score is a promising tool for breast cancer patients undergoing neoadjuvant therapy. We independently evaluated the prognostic value of RCB scores in an extended validation cohort. Additionally, we analyzed the association between chemotherapy dose reduction and RCB scores.
Methods: In this extended validation study, 367 breast cancer patients with available RCB scores were followed up for recurrence-free survival (RFS), distant disease-free survival (DDFS), and overall survival (OS). We also computed standardized cumulative doses of anthracyclines and taxanes (A/Ts) to investigate a potential interaction between neoadjuvant chemotherapy dose reduction and RCB scores.
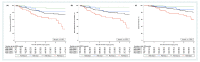
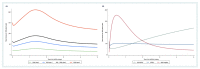
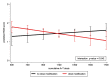
Results: Higher RCB scores were consistently associated with adverse clinical outcomes across different molecular subtypes (HR for RFS = 1.60, 95% CI 1.33-1.93, p < 0.0001; HR for DDFS = 1.70, 95% CI 1.39-2.05, p < 0.0001; HR for OS = 1.67, 95% CI 1.34-2.08, p < 0.0001). The adverse impact prevailed throughout 5 years of follow-up, with a peak for relapse risk between 1-2 years after surgery. Clinical outcomes of patients with RCB class 1 did not differ substantially at 5 years compared to RCB class 0. A total of 180 patients (49.1%) underwent dose reduction of neoadjuvant A/T chemotherapy. We observed a statistically significant interaction between dose reduction and higher RCB scores (interaction p-value = 0.042).
Conclusion: Our results confirm RCB score as a prognostic marker for RFS, DDFS, and OS independent of the molecular subtype. Importantly, we show that lower doses of cumulative neoadjuvant A/T were associated with higher RCB scores in patients who required a dose reduction.
Keywords: RCB; early breast cancer; neoadjuvant systemic therapy.
Conflict of interest statement
C.S. received travel expenses, consulting fees, and honoraria from Amgen, Astra Zeneca, Eli Lilly, Novartis, Pfizer, Roche, and Samsung. M.B. has received honoraria, consulting fees research funds, and/or travel expenses from Amgen, Astra Zeneca, Daichii, Eli Lilly, MSD, Novartis, Pierre-Fabre, Pfizer, Roche, and Samsung. P.J.J. has a consulting or advisory role and received honoraria, research funding, and/or travel/accommodation expenses from Abbvie, Bayer, Boehringer, Novartis, Pfizer, Servier, Roche, BMS, and Celgene.
Figures

References
-
- Broglio K.R., Quintana M., Foster M., Olinger M., McGlothlin A., Berry S.M., Boileau J.F., Brezden-Masley C., Chia S., Dent S., et al. Association of Pathologic Complete Response to Neoadjuvant Therapy in HER2-Positive Breast Cancer With Long-Term Outcomes: A Meta-Analysis. JAMA Oncol. 2016;2:751–760. doi: 10.1001/jamaoncol.2015.6113. - DOI - PubMed
-
- Cortazar P., Zhang L., Untch M., Mehta K., Costantino J.P., Wolmark N., Bonnefoi H., Cameron D., Gianni L., Valagussa P., et al. Pathological complete response and long-term clinical benefit in breast cancer: The CTNeoBC pooled analysis. Lancet. 2014;384:164–172. doi: 10.1016/S0140-6736(13)62422-8. - DOI - PubMed
-
- Von Minckwitz G., Untch M., Blohmer J.U., Costa S.D., Eidtmann H., Fasching P.A., Gerber B., Eiermann W., Hilfrich J., Huober J., et al. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J. Clin. Oncol. 2012;30:1796–1804. doi: 10.1200/JCO.2011.38.8595. - DOI - PubMed
-
- Bossuyt V., Provenzano E., Symmans W.F., Boughey J.C., Coles C., Curigliano G., Dixon J.M., Esserman L.J., Fastner G., Kuehn T., et al. Recommendations for standardized pathological characterization of residual disease for neoadjuvant clinical trials of breast cancer by the BIG-NABCG collaboration. Ann. Oncol. 2015;26:1280–1291. doi: 10.1093/annonc/mdv161. - DOI - PMC - PubMed
-
- Symmans W.F., Peintinger F., Hatzis C., Rajan R., Kuerer H., Valero V., Assad L., Poniecka A., Hennessy B., Green M., et al. Measurement of residual breast cancer burden to predict survival after neoadjuvant chemotherapy. J. Clin. Oncol. 2007;25:4414–4422. doi: 10.1200/JCO.2007.10.6823. - DOI - PubMed
LinkOut - more resources
Full Text Sources

