Long-Term Survival Effect of the Interval between Postoperative Chemotherapy and Radiotherapy in Patients with Completely Resected Pathological N2 Non-Small-Cell Lung Cancer
- PMID: 34065341
- PMCID: PMC8160867
- DOI: 10.3390/cancers13102494
Long-Term Survival Effect of the Interval between Postoperative Chemotherapy and Radiotherapy in Patients with Completely Resected Pathological N2 Non-Small-Cell Lung Cancer
Abstract
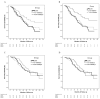
(1) Purpose: To investigate the effects of the time interval between initiation of adjuvant chemotherapy and radiotherapy on survival outcomes in patients with completely resected stage IIIA pN2 non-small-cell lung cancer (NSCLC); (2) Methods: Data on 2515 patients with completely resected stage IIIA pN2 NSCLC in 2007-2017 were extracted from the Taiwan Cancer Registry Database. The survival outcomes in patients who underwent concurrent chemoradiotherapy (CCRT) and sequential chemotherapy and radiotherapy (SCRT) with either a short (SCRT1) or long (SCRT2) interval between treatments were estimated using Kaplan-Meier, Cox regression, and propensity score matching (PSM); (3) Results: Multivariate analyses of OS showed that SCRT2 (hazard ratio [HR] 0.64, p = 0.017) was associated with improved overall survival (OS). After PSM, the median OS periods were 64 and 75 months in the SCRT1 and SCRT2 groups, respectively, which differed significantly from that of 58 months in the CCRT group (p = 0.003). In elderly patients, SCRT2 significantly improved survival relative to CCRT before PSM (p = 0.024) and after PSM (p = 0.002); (4) Conclusions: A longer interval between initiation of adjuvant chemotherapy and postoperative radiotherapy (PORT; SCRT2) improved OS relative to CCRT; the benefits were greater in elderly patients (age >60 years).
Keywords: IMRT; NSCLC; pN2; postoperative chemotherapy; postoperative radiotherapy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Pignon J.E., Tribodet H., Scagliotti G.V., Douillard J., Shepherd F.A., Dunant R.J.S., Torri V., Rosell R., Seymour L., Spiro S.G., et al. Lung adjuvant cisplatin evaluation: A pooled analysis by the LACE Collaborative Group. J. Clin. Oncol. 2008;26:3552–3559. doi: 10.1200/JCO.2007.13.9030. - DOI - PubMed
-
- Arriagada R., Bergman B., Dunant A., le Chevalier T., Pignon J., Vansteenkiste J., International Adjuvant Lung Cancer Trial Collaborative Group Cisplatin-based adjuvant chemotherapy in patients with completely resected non-small-cell lung cancer. N. Engl. J. Med. 2004;350:351–360. doi: 10.1056/nejmoa031644. - DOI - PubMed
Grants and funding
- MOST 107-2634-F-002-016/Ministry of Science and Technology, Taiwan
- MOST 108-2634-F-002-013-/Ministry of Science and Technology, Taiwan
- MOST 109-2634-F-002-028/Ministry of Science and Technology, Taiwan
- MOST 109-2314-B-002-105-MY2/Ministry of Science and Technology, Taiwan
- A1081116/Health Promotion Administration, Ministry of Health and Welfare
LinkOut - more resources
Full Text Sources
Miscellaneous

