Acute Low-Intensity Treadmill Running Upregulates the Expression of Intestinal Glucose Transporters via GLP-2 in Mice
- PMID: 34065342
- PMCID: PMC8160680
- DOI: 10.3390/nu13051735
Acute Low-Intensity Treadmill Running Upregulates the Expression of Intestinal Glucose Transporters via GLP-2 in Mice
Abstract
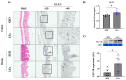
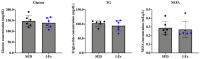
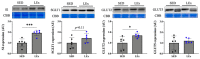
The effects of exercise on nutrient digestion and absorption in the intestinal tract are not well understood. A few studies have reported that exercise training increases the expression of molecules involved in carbohydrate digestion and absorption. Exercise was also shown to increase the blood concentration of glucagon-like peptide-2 (GLP-2), which regulates carbohydrate digestion and absorption in the small intestine. Therefore, we investigated the effects of exercise on the expression of molecules involved in intestinal digestion and absorption, including GLP-2. Six-week-old male mice were divided into a sedentary (SED) and low-intensity exercise (LEx) group. LEx mice were required to run on a treadmill (12.5 m/min, 1 h), whereas SED mice rested. All mice were euthanized 1 h after exercise or rest, and plasma, jejunum, ileum, and colon samples were collected, followed by analysis via IHC, EIA, and immunoblotting. The levels of plasma GLP-2 and the jejunum expression of the GLP-2 receptor, sucrase-isomaltase (SI), and glucose transporter 2 (GLUT2) were higher in LEx mice. Thus, we showed that acute low-intensity exercise affects the expression of molecules involved in intestinal carbohydrate digestion and absorption via GLP-2. Our results suggest that exercise might be beneficial for small intestine function in individuals with intestinal frailty.
Keywords: glucagon-like peptide 2; glucose transporter 2; intestine; low-intensity exercise; sodium-dependent glucose transporter.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Lourenco M.V., Frozza R.L., de Freitas G.B., Zhang H., Kincheski G.C., Ribeiro F.C., Gonçalves R.A., Clarke J.R., Beckman D., Staniszewski A., et al. Exercise-linked FNDC5/irisin rescues synaptic plasticity and memory defects in Alzheimer’s models. Nat. Med. 2019;25:165–175. doi: 10.1038/s41591-018-0275-4. - DOI - PMC - PubMed
-
- Maesako M., Uemura K., Kubota M., Kuzuya A., Sasaki K., Hayashida N., Asada-Utsugi M., Watanabe K., Uemura M., Kihara T., et al. Exercise is more effective than diet control in preventing high fat diet-induced β-amyloid deposition and memory deficit in amyloid precursor protein transgenic mice. J. Biol. Chem. 2012;287:23024–23033. doi: 10.1074/jbc.M112.367011. - DOI - PMC - PubMed
-
- Eshima H., Tamura Y., Kakehi S., Nakamura K., Kurebayashi N., Murayama T., Kakigi R., Sakurai T., Kawamori R., Watada H. Dysfunction of muscle contraction with impaired intracellular Ca2 handling in skeletal muscle and the effect of exercise training in male db/db mice. J. Appl. Physiol. 2019;126:170–182. doi: 10.1152/japplphysiol.00048.2018. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

