Molecular Cloning and Functional Characterization of Heat Stress-Responsive Superoxide Dismutases in Garlic (Allium sativum L.)
- PMID: 34065356
- PMCID: PMC8161062
- DOI: 10.3390/antiox10050815
Molecular Cloning and Functional Characterization of Heat Stress-Responsive Superoxide Dismutases in Garlic (Allium sativum L.)
Abstract
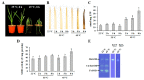
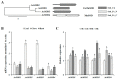
Superoxide dismutases (SODs) are key antioxidant enzymes that can detoxify the superoxide radicals generated by various stresses. Although various plant SODs have been suggested to improve stress tolerance, SODs in garlic, an economically important vegetable grown worldwide, remain relatively unknown. In this study, we found that heat stress strongly induced the activities of Cu/ZnSODs, FeSODs, and MnSODs in garlic leaves. In addition, we cloned four garlic SODs (AsSODs) and suggest that heat stress-increased SOD activity was reflected at least by the induction of these AsSODs. The results of the agro-infiltration assay suggested that the cloned AsSODs encoded functional SOD enzymes belonging to the Cu/ZnSOD and MnSOD families. As a first step toward understanding the enzymatic antioxidant system in garlic plants, our results provide a solid foundation for an in-depth analysis of the physiological functions of the AsSOD family.
Keywords: antioxidant; garlic; heat stress; superoxide dismutase.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Comparative analysis and modeling of superoxide dismutases (SODs) in Brachypodium distachyon L.Appl Biochem Biotechnol. 2014 Jul;173(5):1183-96. doi: 10.1007/s12010-014-0922-2. Epub 2014 Apr 30. Appl Biochem Biotechnol. 2014. PMID: 24781980
-
Molecular evolution and structure--function relationships of the superoxide dismutase gene families in angiosperms and their relationship to other eukaryotic and prokaryotic superoxide dismutases.Arch Biochem Biophys. 2002 Mar 1;399(1):19-36. doi: 10.1006/abbi.2001.2739. Arch Biochem Biophys. 2002. PMID: 11883900
-
The molecular characterizations of Cu/ZnSOD and MnSOD and its responses of mRNA expression and enzyme activity to Aeromonas hydrophila or lipopolysaccharide challenge in Qihe crucian carp Carassius auratus.Fish Shellfish Immunol. 2017 Aug;67:429-440. doi: 10.1016/j.fsi.2017.06.031. Epub 2017 Jun 10. Fish Shellfish Immunol. 2017. PMID: 28606861
-
Characterization of two copper/zinc superoxide dismutases (Cu/Zn-SODs) from the desert beetle Microdera punctipennis and their activities in protecting E. coli cells against cold.Cryobiology. 2019 Apr;87:15-27. doi: 10.1016/j.cryobiol.2019.03.006. Epub 2019 Mar 16. Cryobiology. 2019. PMID: 30890324 Review.
-
Expanding roles of superoxide dismutases in cell regulation and cancer.Drug Discov Today. 2016 Jan;21(1):143-149. doi: 10.1016/j.drudis.2015.10.001. Epub 2015 Oct 19. Drug Discov Today. 2016. PMID: 26475962 Free PMC article. Review.
Cited by
-
PlgMYBR1, an R2R3-MYB transcription factor, plays as a negative regulator of anthocyanin biosynthesis in Platycodon grandiflorus.3 Biotech. 2023 Mar;13(3):75. doi: 10.1007/s13205-023-03490-6. Epub 2023 Feb 3. 3 Biotech. 2023. PMID: 36748016 Free PMC article.
-
Insilco identification and characterization of superoxide dismutase gene family in Brassica rapa.Saudi J Biol Sci. 2021 Oct;28(10):5526-5537. doi: 10.1016/j.sjbs.2021.08.009. Epub 2021 Aug 8. Saudi J Biol Sci. 2021. PMID: 34588862 Free PMC article.
-
The Superoxide Dismutase Family in Balloon Flower (Platycodon grandiflorus): Phylogenetic Relationships, Structural Characteristics, and Expression Patterns.Curr Issues Mol Biol. 2025 May 12;47(5):351. doi: 10.3390/cimb47050351. Curr Issues Mol Biol. 2025. PMID: 40699750 Free PMC article.
-
Physiological and sucrose metabolic responses to waterlogging stress in balloon flower (Platycodon grandiflorus (Jacq.) A. DC).Physiol Mol Biol Plants. 2023 Apr;29(4):591-600. doi: 10.1007/s12298-023-01310-y. Epub 2023 Apr 29. Physiol Mol Biol Plants. 2023. PMID: 37181045 Free PMC article.
-
New seed coating containing Trichoderma viride with anti-pathogenic properties.PeerJ. 2023 Jun 1;11:e15392. doi: 10.7717/peerj.15392. eCollection 2023. PeerJ. 2023. PMID: 37283892 Free PMC article.
References
-
- Hasanuzzaman M., Bhuyan M.H.M., Zulfiqar F., Raza A., Mohsin S.M., Mahmud J.A., Fujita M., Fotopoulos V. Reactive oxygen species and antioxidant defense in plants under abiotic stress: Revisiting the crucial role of a universal defense regulator. Antioxidants. 2020;9:681. doi: 10.3390/antiox9080681. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

