SARS-CoV-2 Disinfection of Air and Surface Contamination by TiO2 Photocatalyst-Mediated Damage to Viral Morphology, RNA, and Protein
- PMID: 34065382
- PMCID: PMC8161138
- DOI: 10.3390/v13050942
SARS-CoV-2 Disinfection of Air and Surface Contamination by TiO2 Photocatalyst-Mediated Damage to Viral Morphology, RNA, and Protein
Abstract
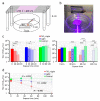
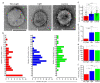
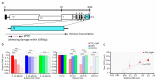
SARS-CoV-2 is the causative agent of COVID-19, which is a global pandemic. SARS-CoV-2 is transmitted rapidly via contaminated surfaces and aerosols, emphasizing the importance of environmental disinfection to block the spread of virus. Ultraviolet C radiation and chemical compounds are effective for SARS-CoV-2 disinfection, but can only be applied in the absence of humans due to their toxicities. Therefore, development of disinfectants that can be applied in working spaces without evacuating people is needed. Here we showed that TiO2-mediated photocatalytic reaction inactivates SARS-CoV-2 in a time-dependent manner and decreases its infectivity by 99.9% after 20 min and 120 min of treatment in aerosol and liquid, respectively. The mechanistic effects of TiO2 photocatalyst on SARS-CoV-2 virion included decreased total observed virion count, increased virion size, and reduced particle surface spike structure, as determined by transmission electron microscopy. Damage to viral proteins and genome was further confirmed by western blotting and RT-qPCR, respectively. The multi-antiviral effects of TiO2-mediated photocatalytic reaction implies universal disinfection potential for different infectious agents. Notably, TiO2 has no adverse effects on human health, and therefore, TiO2-induced photocatalytic reaction is suitable for disinfection of SARS-CoV-2 and other emerging infectious disease-causing agents in human habitation.
Keywords: RNA damage; SARS-CoV-2 inactivation; TiO2 photocatalyst; aerosol; viral morphology disruption; viral protein damage.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Naqvi A.A.T., Fatima K., Mohammad T., Fatima U., Singh I.K., Singh A., Atif S.M., Hariprasad G., Hasan G.M., Hassan M.I. Insights into SARS-CoV-2 genome, structure, evolution, pathogenesis and therapies: Structural genomics approach. Biochim. Biophys. Acta Mol. Basis. Dis. 2020;1866:165878. doi: 10.1016/j.bbadis.2020.165878. - DOI - PMC - PubMed
-
- van Doremalen N., Bushmaker T., Morris D.H., Holbrook M.G., Gamble A., Williamson B.N., Tamin A., Harcourt J.L., Thornburg N.J., Gerber S.I., et al. Aerosol and Surface Stability of SARS-CoV-2 as Compared with SARS-CoV-1. N. Engl. J. Med. 2020;382:1564–1567. doi: 10.1056/NEJMc2004973. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

