Retinal Pigment Epithelium Remodeling in Mouse Models of Retinitis Pigmentosa
- PMID: 34065385
- PMCID: PMC8161377
- DOI: 10.3390/ijms22105381
Retinal Pigment Epithelium Remodeling in Mouse Models of Retinitis Pigmentosa
Abstract
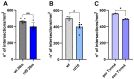
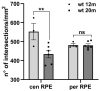
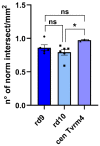
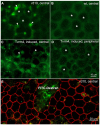
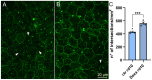
In retinitis pigmentosa (RP), one of many possible genetic mutations causes rod degeneration, followed by cone secondary death leading to blindness. Accumulating evidence indicates that rod death triggers multiple, non-cell-autonomous processes, which include oxidative stress and inflammation/immune responses, all contributing to cone demise. Inflammation relies on local microglia and recruitment of immune cells, reaching the retina through breakdowns of the inner blood retinal barrier (iBRB). Leakage in the inner retina vasculature suggests similarly altered outer BRB, formed by junctions between retinal pigment epithelium (RPE) cells, which are crucial for retinal homeostasis, immune response, and privilege. We investigated the RPE structural integrity in three models of RP (rd9, rd10, and Tvrm4 mice) by immunostaining for zonula occludens-1 (ZO-1), an essential regulatory component of tight junctions. Quantitative image analysis demonstrated discontinuities in ZO-1 profiles in all mutants, despite different degrees of photoreceptor loss. ZO-1 interruption zones corresponded to leakage of in vivo administered, fluorescent dextran through the choroid-RPE interface, demonstrating barrier dysfunction. Dexamethasone, administered to rd10 mice for rescuing cones, also rescued RPE structure. Thus, previously undetected, stereotyped abnormalities occur in the RPE of RP mice; pharmacological targeting of inflammation supports a feedback loop leading to simultaneous protection of cones and the RPE.
Keywords: Tvrm4 mouse; blood retinal barrier; dextranes; immunocytochemistry; rd10 mouse; rd9 mouse; retinal pigment epithelium; retinitis pigmentosa; zonula occludens.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; the collection, analyses, or interpretation of data; the writing of the manuscript; or in the decision to publish the results.
Figures





Similar articles
-
Wheel running exercise protects against retinal degeneration in the I307N rhodopsin mouse model of inducible autosomal dominant retinitis pigmentosa.Mol Vis. 2019 Aug 21;25:462-476. eCollection 2019. Mol Vis. 2019. PMID: 31523123 Free PMC article.
-
Structural abnormalities of retinal pigment epithelial cells in a light-inducible, rhodopsin mutant mouse.J Anat. 2023 Aug;243(2):223-234. doi: 10.1111/joa.13667. Epub 2022 Apr 15. J Anat. 2023. PMID: 35428980 Free PMC article.
-
Targeting the tight junction protein, zonula occludens-1, with the connexin43 mimetic peptide, αCT1, reduces VEGF-dependent RPE pathophysiology.J Mol Med (Berl). 2017 May;95(5):535-552. doi: 10.1007/s00109-017-1506-8. Epub 2017 Jan 28. J Mol Med (Berl). 2017. PMID: 28132078 Free PMC article.
-
Using the rd1 mouse to understand functional and anatomical retinal remodelling and treatment implications in retinitis pigmentosa: A review.Exp Eye Res. 2016 Sep;150:106-21. doi: 10.1016/j.exer.2015.10.019. Epub 2015 Oct 30. Exp Eye Res. 2016. PMID: 26521764 Review.
-
Is Retinal Metabolic Dysfunction at the Center of the Pathogenesis of Age-related Macular Degeneration?Int J Mol Sci. 2019 Feb 11;20(3):762. doi: 10.3390/ijms20030762. Int J Mol Sci. 2019. PMID: 30754662 Free PMC article. Review.
Cited by
-
Txnip deletions and missense alleles prolong the survival of cones in a retinitis pigmentosa mouse model.Elife. 2024 May 10;12:RP90749. doi: 10.7554/eLife.90749. Elife. 2024. PMID: 38727583 Free PMC article.
-
Targeting miR-181a/b in retinitis pigmentosa: implications for disease progression and therapy.Cell Biosci. 2024 May 21;14(1):64. doi: 10.1186/s13578-024-01243-3. Cell Biosci. 2024. PMID: 38773556 Free PMC article.
-
Insight into the role of non-coding RNA in the diagnosis and treatment of retinitis pigmentosa.Noncoding RNA Res. 2023 Oct 29;9(1):44-54. doi: 10.1016/j.ncrna.2023.10.011. eCollection 2024 Mar. Noncoding RNA Res. 2023. PMID: 38075200 Free PMC article. Review.
-
Decitabine improves MMS-induced retinal photoreceptor cell damage by targeting DNMT3A and DNMT3B.Front Mol Neurosci. 2023 Jan 10;15:1057365. doi: 10.3389/fnmol.2022.1057365. eCollection 2022. Front Mol Neurosci. 2023. PMID: 36704326 Free PMC article.
-
Advances in the engineering of the outer blood-retina barrier: From in-vitro modelling to cellular therapy.Bioact Mater. 2023 Aug 12;31:151-177. doi: 10.1016/j.bioactmat.2023.08.003. eCollection 2024 Jan. Bioact Mater. 2023. PMID: 37637086 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

