Unicellular versus Filamentous: The Glacial Alga Ancylonema alaskana comb. et stat. nov. and Its Ecophysiological Relatedness to Ancylonema nordenskioeldii (Zygnematophyceae, Streptophyta)
- PMID: 34065466
- PMCID: PMC8161032
- DOI: 10.3390/microorganisms9051103
Unicellular versus Filamentous: The Glacial Alga Ancylonema alaskana comb. et stat. nov. and Its Ecophysiological Relatedness to Ancylonema nordenskioeldii (Zygnematophyceae, Streptophyta)
Abstract
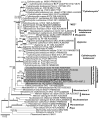
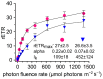
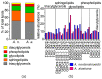
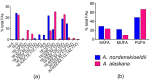
Melting polar and alpine ice surfaces frequently exhibit blooms of dark pigmented algae. These microbial extremophiles significantly reduce the surface albedo of glaciers, thus accelerating melt rates. However, the ecology, physiology and taxonomy of cryoflora are not yet fully understood. Here, a Swiss and an Austrian glacier dominated either by filamentous Ancylonema nordenskioeldii or unicellular Mesotaenium berggrenii var. alaskanum, were sampled. Molecular analysis showed that both species are closely related, sharing identical chloroplast morphologies (parietal-lobed for Ancylonema vs. axial plate-like for Mesotaenium sensu stricto), thus the unicellular species was renamed Ancylonema alaskana. Moreover, an ecophysiological comparison of the two species was performed: pulse-amplitude modulated (PAM) fluorometry confirmed that they have a high tolerance to elevated solar irradiation, the physiological light preferences reflected the conditions in the original habitat; nonetheless, A. nordenskioeldii was adapted to higher irradiances while the photosystems of A. alaskana were able to use efficiently low irradiances. Additionally, the main vacuolar polyphenol, which effectively shields the photosystems, was identical in both species. Also, about half of the cellular fatty acids were polyunsaturated, and the lipidome profiles dominated by triacylglycerols were very similar. The results indicate that A. alaskana is physiologically very similar and closely related but genetically distinct to A. nordenskioeldii.
Keywords: Mesotaeniaceae; cryoflora; fatty acids; lipidome; photosynthesis; phylogeny; polyphenols; supraglacial communities.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures


References
-
- Ling H.U., Seppelt R.D. Snow algae of the Windmill Islands, continental Antarctica. Mesotaenium berggrenii (Zygnematales, Chlorophyta) the alga of grey snow. Antarct. Sci. 1990;2:143–148. doi: 10.1007/s003000050309. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

