Early-Pregnancy Dydrogesterone Supplementation Mimicking Luteal-Phase Support in ART Patients Did Not Provoke Major Reproductive Disorders in Pregnant Mice and Their Progeny
- PMID: 34065597
- PMCID: PMC8161261
- DOI: 10.3390/ijms22105403
Early-Pregnancy Dydrogesterone Supplementation Mimicking Luteal-Phase Support in ART Patients Did Not Provoke Major Reproductive Disorders in Pregnant Mice and Their Progeny
Abstract
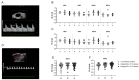
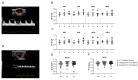
Progestogens are frequently administered during early pregnancy to patients undergoing assisted reproductive techniques (ART) to overcome progesterone deficits following ART procedures. Orally administered dydrogesterone (DG) shows equal efficacy to other progestogens with a higher level of patient compliance. However, potential harmful effects of DG on critical pregnancy processes and on the health of the progeny are not yet completely ruled out. We treated pregnant mice with DG in the mode, duration, and doses comparable to ART patients. Subsequently, we studied DG effects on embryo implantation, placental and fetal growth, fetal-maternal circulation, fetal survival, and the uterine immune status. After birth of in utero DG-exposed progeny, we assessed their sex ratios, weight gain, and reproductive performance. Early-pregnancy DG administration did not interfere with placental and fetal development, fetal-maternal circulation, or fetal survival, and provoked only minor changes in the uterine immune compartment. DG-exposed offspring grew normally, were fertile, and showed no reproductive abnormalities with the exception of an altered spermiogram in male progeny. Notably, DG shifted the sex ratio in favor of female progeny. Even though our data may be reassuring for the use of DG in ART patients, the detrimental effects on spermatogenesis in mice warrants further investigations and may be a reason for caution for routine DG supplementation in early pregnancy.
Keywords: artificial reproductive techniques; dydrogesterone; early pregnancy; luteal-phase support; progeny; reproductive disorders; safety; tolerability.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures



Similar articles
-
Oral dydrogesterone versus intravaginal micronized progesterone gel for luteal phase support in IVF: a randomized clinical trial.Hum Reprod. 2018 Dec 1;33(12):2212-2221. doi: 10.1093/humrep/dey306. Hum Reprod. 2018. PMID: 30304457 Free PMC article. Clinical Trial.
-
Oral dydrogesterone versus intravaginal micronised progesterone as luteal phase support in assisted reproductive technology (ART) cycles: results of a randomised study.J Steroid Biochem Mol Biol. 2005 Dec;97(5):416-20. doi: 10.1016/j.jsbmb.2005.08.012. Epub 2005 Oct 5. J Steroid Biochem Mol Biol. 2005. PMID: 16213136 Clinical Trial.
-
Live birth rates and safety profile using dydrogesterone for luteal phase support in assisted reproductive techniques.Singapore Med J. 2017 Jun;58(6):294-297. doi: 10.11622/smedj.2016080. Epub 2016 Apr 19. Singapore Med J. 2017. PMID: 27090598 Free PMC article.
-
Dydrogesterone: pharmacological profile and mechanism of action as luteal phase support in assisted reproduction.Reprod Biomed Online. 2019 Feb;38(2):249-259. doi: 10.1016/j.rbmo.2018.11.017. Epub 2018 Dec 15. Reprod Biomed Online. 2019. PMID: 30595525 Review.
-
Dydrogesterone as an oral alternative to vaginal progesterone for IVF luteal phase support: A systematic review and individual participant data meta-analysis.PLoS One. 2020 Nov 4;15(11):e0241044. doi: 10.1371/journal.pone.0241044. eCollection 2020. PLoS One. 2020. PMID: 33147288 Free PMC article.
Cited by
-
Evaluation of the Efficacy and Adverse Reactions of Mirena Combined with Hysteroscopic Surgery When Treating AUB: Based on a Retrospective Cohort Study.Comput Math Methods Med. 2022 Jun 11;2022:4082266. doi: 10.1155/2022/4082266. eCollection 2022. Comput Math Methods Med. 2022. Retraction in: Comput Math Methods Med. 2023 Sep 27;2023:9898345. doi: 10.1155/2023/9898345. PMID: 35726229 Free PMC article. Retracted.
-
Meta analysis of the effect of phloroglucinol combined with progesterone in the treatment of threatened miscarriage before 20 weeks of gestation: A protocol for a systematic review.Medicine (Baltimore). 2022 Nov 25;101(47):e31885. doi: 10.1097/MD.0000000000031885. Medicine (Baltimore). 2022. PMID: 36451473 Free PMC article.
-
The Role of Dydrogesterone in the Management of Luteal Phase Defect: A Comprehensive Review.Cureus. 2023 Nov 3;15(11):e48194. doi: 10.7759/cureus.48194. eCollection 2023 Nov. Cureus. 2023. PMID: 38050524 Free PMC article. Review.
-
The ENDOMIX perspective: how everyday chemical mixtures impact human health and reproduction by targeting the immune system†.Biol Reprod. 2024 Dec 12;111(6):1170-1187. doi: 10.1093/biolre/ioae142. Biol Reprod. 2024. PMID: 39446589 Free PMC article. Review.
References
-
- Barbosa M.W.P., Valadares N.P.B., Barbosa A.C.P., Amaral A.S., Iglesias J.R., Nastri C.O., Martins W.P., Nakagawa H.M. Oral dydrogesterone vs. vaginal progesterone capsules for luteal-phase support in women undergoing embryo transfer: A systematic review and meta-analysis. JBRA Assist. Reprod. 2018;22:148–156. doi: 10.5935/1518-0557.20180018. - DOI - PMC - PubMed
-
- Griesinger G., Blockeel C., Kahler E., Pexman-Fieth C., Olofsson J.I., Driessen S., Tournaye H. Dydrogesterone as an oral alternative to vaginal progesterone for IVF luteal phase support: A systematic review and individual participant data meta-analysis. PLoS ONE. 2020;15:e0241044. doi: 10.1371/journal.pone.0241044. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

