Green Tea Epigallocatechin-3-gallate (EGCG) Targeting Protein Misfolding in Drug Discovery for Neurodegenerative Diseases
- PMID: 34065606
- PMCID: PMC8160836
- DOI: 10.3390/biom11050767
Green Tea Epigallocatechin-3-gallate (EGCG) Targeting Protein Misfolding in Drug Discovery for Neurodegenerative Diseases
Abstract
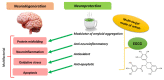
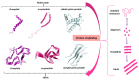
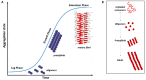
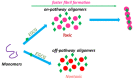
The potential to treat neurodegenerative diseases (NDs) of the major bioactive compound of green tea, epigallocatechin-3-gallate (EGCG), is well documented. Numerous findings now suggest that EGCG targets protein misfolding and aggregation, a common cause and pathological mechanism in many NDs. Several studies have shown that EGCG interacts with misfolded proteins such as amyloid beta-peptide (Aβ), linked to Alzheimer's disease (AD), and α-synuclein, linked to Parkinson's disease (PD). To date, NDs constitute a serious public health problem, causing a financial burden for health care systems worldwide. Although current treatments provide symptomatic relief, they do not stop or even slow the progression of these devastating disorders. Therefore, there is an urgent need to develop effective drugs for these incurable ailments. It is expected that targeting protein misfolding can serve as a therapeutic strategy for many NDs since protein misfolding is a common cause of neurodegeneration. In this context, EGCG may offer great potential opportunities in drug discovery for NDs. Therefore, this review critically discusses the role of EGCG in NDs drug discovery and provides updated information on the scientific evidence that EGCG can potentially be used to treat many of these fatal brain disorders.
Keywords: Alzheimer’s disease; Parkinson’s disease; amyloid-β; anti-amyloidogenic; anti-neurodegenerative; catechins; epigallocatechin-3-gallate; misfolded proteins; natural products; neuroprotective; α-synuclein.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Dorsey E.R., Elbaz A., Nichols E., Abd-Allah F., Abdelalim A., Adsuar J.C., Ansha M.G., Brayne C., Choi J.Y.J., Collado-Mateo D., et al. Global, regional, and national burden of Parkinson’s disease, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2018;17:939–953. doi: 10.1016/S1474-4422(18)30295-3. - DOI - PMC - PubMed
-
- Nichols E., Szoeke C.E.I., Vollset S.E., Abbasi N., Abd-Allah F., Abdela J., Aichour M.T.E., Akinyemi R.O., Alahdab F., Asgedom S.W., et al. Global, regional, and national burden of Alzheimer’s disease and other dementias, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18:88–106. doi: 10.1016/S1474-4422(18)30403-4. - DOI - PMC - PubMed
-
- Yacoubian T.A. Neurodegenerative Disorders: Why Do We Need New Therapies? Elsevier Inc.; Amsterdam, The Netherlands: 2017.
Publication types
MeSH terms
Substances
Grants and funding
- E-26/202.625/2019-BOLSA/Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro
- E26/010.002128/2019/Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro
- Finance Code 001/Coordenação de Aperfeiçoamento de Pessoal de Nível Superior
- 307294/2018-8/Conselho Nacional de Desenvolvimento Científico e Tecnológico
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

