Methionine Sulfoxide Reductases Contribute to Anaerobic Fermentative Metabolism in Bacillus cereus
- PMID: 34065610
- PMCID: PMC8161402
- DOI: 10.3390/antiox10050819
Methionine Sulfoxide Reductases Contribute to Anaerobic Fermentative Metabolism in Bacillus cereus
Abstract
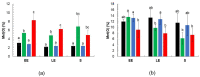
Reversible oxidation of methionine to methionine sulfoxide (Met(O)) is a common posttranslational modification occurring on proteins in all organisms under oxic conditions. Protein-bound Met(O) is reduced by methionine sulfoxide reductases, which thus play a significant antioxidant role. The facultative anaerobe Bacillus cereus produces two methionine sulfoxide reductases: MsrA and MsrAB. MsrAB has been shown to play a crucial physiological role under oxic conditions, but little is known about the role of MsrA. Here, we examined the antioxidant role of both MsrAB and MrsA under fermentative anoxic conditions, which are generally reported to elicit little endogenous oxidant stress. We created single- and double-mutant Δmsr strains. Compared to the wild-type and ΔmsrAB mutant, single- (ΔmsrA) and double- (ΔmsrAΔmsrAB) mutants accumulated higher levels of Met(O) proteins, and their cellular and extracellular Met(O) proteomes were altered. The growth capacity and motility of mutant strains was limited, and their energy metabolism was altered. MsrA therefore appears to play a major physiological role compared to MsrAB, placing methionine sulfoxides at the center of the B. cereus antioxidant system under anoxic fermentative conditions.
Keywords: Bacillus cereus; anaerobiosis; methionine oxidation; methionine sulfoxide reductase.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Methionine Residues in Exoproteins and Their Recycling by Methionine Sulfoxide Reductase AB Serve as an Antioxidant Strategy in Bacillus cereus.Front Microbiol. 2017 Jul 26;8:1342. doi: 10.3389/fmicb.2017.01342. eCollection 2017. Front Microbiol. 2017. PMID: 28798727 Free PMC article.
-
Spx mediates oxidative stress regulation of the methionine sulfoxide reductases operon in Bacillus subtilis.BMC Microbiol. 2008 Jul 28;8:128. doi: 10.1186/1471-2180-8-128. BMC Microbiol. 2008. PMID: 18662407 Free PMC article.
-
Methionine sulfoxide reductases are essential for virulence of Salmonella typhimurium.PLoS One. 2011;6(11):e26974. doi: 10.1371/journal.pone.0026974. Epub 2011 Nov 2. PLoS One. 2011. PMID: 22073230 Free PMC article.
-
Methionine sulfoxide reductases and virulence of bacterial pathogens.Future Microbiol. 2007 Dec;2(6):619-30. doi: 10.2217/17460913.2.6.619. Future Microbiol. 2007. PMID: 18041903 Review.
-
Origin and evolution of the protein-repairing enzymes methionine sulphoxide reductases.Biol Rev Camb Philos Soc. 2008 Aug;83(3):249-57. doi: 10.1111/j.1469-185X.2008.00042.x. Biol Rev Camb Philos Soc. 2008. PMID: 18557976 Review.
Cited by
-
The Resistance Abilities of Some Bacillus Species to Gastrointestinal Tract Conditions: Whole Genome Sequencing of the Novel Candidate Probiotic Strains Bacillus clausiiBA8 and Bacillus subtilisBA11.Food Sci Nutr. 2025 Feb 5;13(2):e70018. doi: 10.1002/fsn3.70018. eCollection 2025 Feb. Food Sci Nutr. 2025. PMID: 39911839 Free PMC article.
-
Methionine oxidation under anaerobic conditions in Escherichia coli.Mol Microbiol. 2022 Oct;118(4):387-402. doi: 10.1111/mmi.14971. Epub 2022 Aug 17. Mol Microbiol. 2022. PMID: 36271735 Free PMC article.
-
Region Met225 to Ile412 of Bacillus cereus Hemolysin II Is Capable to Agglutinate Red Blood Cells.Molecules. 2023 Apr 19;28(8):3581. doi: 10.3390/molecules28083581. Molecules. 2023. PMID: 37110815 Free PMC article.
-
Pan-genome-scale metabolic modeling of Bacillus subtilis reveals functionally distinct groups.mSystems. 2024 Nov 19;9(11):e0092324. doi: 10.1128/msystems.00923-24. Epub 2024 Oct 4. mSystems. 2024. PMID: 39365060 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

