BMAL1 Knockdown Leans Epithelial-Mesenchymal Balance toward Epithelial Properties and Decreases the Chemoresistance of Colon Carcinoma Cells
- PMID: 34065633
- PMCID: PMC8157026
- DOI: 10.3390/ijms22105247
BMAL1 Knockdown Leans Epithelial-Mesenchymal Balance toward Epithelial Properties and Decreases the Chemoresistance of Colon Carcinoma Cells
Abstract
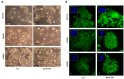
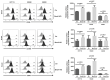
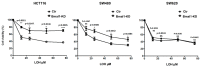
The circadian clock coordinates biological and physiological functions to day/night cycles. The perturbation of the circadian clock increases cancer risk and affects cancer progression. Here, we studied how BMAL1 knockdown (BMAL1-KD) by shRNA affects the epithelial-mesenchymal transition (EMT), a critical early event in the invasion and metastasis of colorectal carcinoma (CRC). In corresponding to a gene set enrichment analysis, which showed a significant enrichment of EMT and invasive signatures in BMAL1_high CRC patients as compared to BMAL1_low CRC patients, our results revealed that BMAL1 is implicated in keeping the epithelial-mesenchymal equilibrium of CRC cells and influences their capacity of adhesion, migration, invasion, and chemoresistance. Firstly, BMAL1-KD increased the expression of epithelial markers (E-cadherin, CK-20, and EpCAM) but decreased the expression of Twist and mesenchymal markers (N-cadherin and vimentin) in CRC cell lines. Finally, the molecular alterations after BMAL1-KD promoted mesenchymal-to-epithelial transition-like changes mostly appeared in two primary CRC cell lines (i.e., HCT116 and SW480) compared to the metastatic cell line SW620. As a consequence, migration/invasion and drug resistance capacities decreased in HCT116 and SW480 BMAL1-KD cells. Together, BMAL1-KD alerts the delicate equilibrium between epithelial and mesenchymal properties of CRC cell lines, which revealed the crucial role of BMAL1 in EMT-related CRC metastasis and chemoresistance.
Keywords: BMAL1; chemoresistance; circadian clock; colorectal cancer; epithelial–mesenchymal transition (EMT); metastasis.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures




References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

