Amino Acid Substitutions in NS5 Contribute Differentially to Tembusu Virus Attenuation in Ducklings and Cell Cultures
- PMID: 34065634
- PMCID: PMC8156267
- DOI: 10.3390/v13050921
Amino Acid Substitutions in NS5 Contribute Differentially to Tembusu Virus Attenuation in Ducklings and Cell Cultures
Abstract
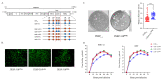
Tembusu virus (TMUV), a highly infectious pathogenic flavivirus, causes severe egg-drop and encephalitis in domestic waterfowl, while the determinants responsible for viral pathogenicity are largely unknown. In our previous studies, virulent strain JXSP2-4 had been completely attenuated by successive passages in BHK-21 cells and the avirulent strain was designated as JXSP-310. Based on the backbone of JXSP2-4, a series of chimeric viruses were generated according to the amino acid substitutions in NS5 and their infectivities were also analyzed in cell cultures and ducklings. The results showed that the viral titers of RNA-dependent RNA polymerase (RdRp) domain-swapped cheimeric mutant (JXSP-310RdRp) in cells and ducklings were both markedly decreased compared with JXSP2-4, indicating that mutations in the RdRp domain affected viral replication. There are R543K and V711A two amino acid substitutions in the RdRp domain. Further site-directed mutagenesis showed that single-point R543K mutant (JXSP-R543K) exhibited similar replication efficacy compared with JXSP2-4 in cells, but the viral loads in JXSP-R543K-infected ducklings were significantly lower than that of JXSP2-4 and higher than JXSP-310RdRp. Surprisingly, the single-point V711A mutation we introduced rapidly reverted. In addition, qRT-PCR and Western blot confirmed that the mutations in the RdRp domain significantly affected the replication of the virus. Taken together, these results show that R543K substitution in the RdRp domain impairs the in vivo growth of TMUV, but sustaining its attenuated infectivity requires the concurrent presence of the V711A mutation.
Keywords: RNA-dependent RNA polymerase; Tembusu virus; attenuation; chimeric viruses; viral replication.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Liu P., Lu H., Li S., Moureau G., Deng Y.Q., Wang Y., Zhang L., Jiang T., de Lamballerie X., Qin C.F., et al. Genomic and antigenic characterization of the newly emerging Chinese duck egg-drop syndrome flavivirus: Genomic comparison with Tembusu and Sitiawan viruses. J. Gen. Virol. 2012;93:2158–2170. doi: 10.1099/vir.0.043554-0. - DOI - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

