COVID-19 Vaccine: A Survey of Hesitancy in Patients with Celiac Disease
- PMID: 34065654
- PMCID: PMC8156726
- DOI: 10.3390/vaccines9050511
COVID-19 Vaccine: A Survey of Hesitancy in Patients with Celiac Disease
Abstract
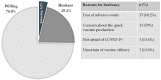
(1) Background: COVID-19 vaccination campaigns offer the best hope of controlling the pandemic. However, the fast production of COVID-19 vaccines has caused concern among the general public regarding their safety and efficacy. In particular, patients with chronic illnesses, such as celiac disease (CD), may be more fearful. Information on vaccine hesitancy plays a pivotal role in the development of an efficient vaccination campaign. In our study, we aimed to evaluate COVID-19 vaccine hesitancy among Italian CD patients. (2) Methods: an anonymous questionnaire was sent to CD patients followed at our tertiary referral center for CD in Milan, Italy. Patients were defined as willing, hesitant and refusing. We evaluated the reasons for hesitancy/refusal and the possible determinants, calculating crude and adjusted odds ratios [AdjORs] with 95% confidence intervals [CIs]. (3) Results: the questionnaire was sent to 346 patients with a response rate of 29.8%. Twenty-six (25.2%) of the 103 respondents were hesitant, with a total refusal rate of 4.8%. The main reason was fear of adverse events related to vaccination (68.2%). Among hesitant patients, 23% declared that their opinion was influenced by their CD. The determinants positively influencing willingness to be vaccinated against COVID-19 were adherence to a GFD, perception of good knowledge about COVID-19 and its vaccines, and a positive attitude to previous vaccines (AdjOR 12.71, 95% CI 1.82-88.58, AdjOR 6.50, 95% CI 1.44-29.22, AdjOR 0.70, 95% CI 0.11-4.34, respectively). (4) Conclusions: CD patients should be vaccinated against COVID-19 and a specific campaign to address the determinants of hesitancy should be developed.
Keywords: COVID-19; COVID-19 vaccines; celiac disease; vaccine hesitancy; vaccines.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
COVID-19 Vaccine Acceptance among Liver Transplant Recipients.Vaccines (Basel). 2021 Nov 11;9(11):1314. doi: 10.3390/vaccines9111314. Vaccines (Basel). 2021. PMID: 34835245 Free PMC article.
-
Vaccination Status and Attitudes towards Vaccines in a Cohort of Patients with Celiac Disease.Vaccines (Basel). 2022 Jul 27;10(8):1199. doi: 10.3390/vaccines10081199. Vaccines (Basel). 2022. PMID: 36016087 Free PMC article.
-
Public Willingness and Determinants of COVID-19 Vaccination at the Initial Stage of Mass Vaccination in China.Vaccines (Basel). 2021 Oct 13;9(10):1172. doi: 10.3390/vaccines9101172. Vaccines (Basel). 2021. PMID: 34696281 Free PMC article.
-
"First Do No Harm". No-Fault Compensation Program for COVID-19 Vaccines as Feasibility and Wisdom of a Policy Instrument to Mitigate Vaccine Hesitancy.Vaccines (Basel). 2021 Sep 30;9(10):1116. doi: 10.3390/vaccines9101116. Vaccines (Basel). 2021. PMID: 34696224 Free PMC article. Review.
-
COVID-19 Vaccines: A Radiological Review of the Good, the Bad, and the Ugly.Indian J Radiol Imaging. 2024 Apr 21;34(4):714-725. doi: 10.1055/s-0044-1785210. eCollection 2024 Oct. Indian J Radiol Imaging. 2024. PMID: 39318578 Free PMC article. Review.
Cited by
-
Changes in COVID-19 Perception and in TMD Prevalence after 1 Year of Pandemic in Italy.Eur J Dent. 2023 Jul;17(3):771-776. doi: 10.1055/s-0042-1755192. Epub 2022 Sep 20. Eur J Dent. 2023. PMID: 36126959 Free PMC article.
-
COVID-19 Vaccine Acceptance among Liver Transplant Recipients.Vaccines (Basel). 2021 Nov 11;9(11):1314. doi: 10.3390/vaccines9111314. Vaccines (Basel). 2021. PMID: 34835245 Free PMC article.
-
COVID-19 Vaccination in a Patient With Gluten Enteropathy: A Case Report.Cureus. 2024 Feb 6;16(2):e53738. doi: 10.7759/cureus.53738. eCollection 2024 Feb. Cureus. 2024. PMID: 38465180 Free PMC article.
-
Attitudes, acceptance and hesitancy among the general population worldwide to receive the COVID-19 vaccines and their contributing factors: A systematic review.EClinicalMedicine. 2021 Oct;40:101113. doi: 10.1016/j.eclinm.2021.101113. Epub 2021 Sep 2. EClinicalMedicine. 2021. PMID: 34490416 Free PMC article.
-
Emergency Approval Mechanisms for Human Vaccines in India.Pharmaceut Med. 2024 Mar;38(2):121-132. doi: 10.1007/s40290-023-00513-8. Epub 2024 Jan 24. Pharmaceut Med. 2024. PMID: 38265630 Review.
References
-
- COVID-19 Map—Johns Hopkins Coronavirus Resource Center. [(accessed on 26 April 2021)]; Available online: https://coronavirus.jhu.edu/map.html.
-
- Agenzia Italiana del Farmaco Vaccini COVID-19. [(accessed on 26 April 2021)]; Available online: https://www.aifa.gov.it/vaccini-covid-19.
-
- European Medicines Agency Treatments and vaccines for COVID-19. [(accessed on 26 April 2021)]; Available online: https://www.ema.europa.eu/en/human-regulatory/overview/public-health-thr....
-
- FDA COVID-19 Vaccines: The FDA Has Regulatory Processes in Place to Facilitate the Development of COVID-19 Vaccines that Meet the FDA’s Rigorous Scientific Standards. [(accessed on 26 April 2021)]; Available online: https://www.fda.gov/emergency-preparedness-and-response/coronavirus-dise....