2D Porphyrinic Metal-Organic Frameworks Featuring Rod-Shaped Secondary Building Units
- PMID: 34065664
- PMCID: PMC8156857
- DOI: 10.3390/molecules26102955
2D Porphyrinic Metal-Organic Frameworks Featuring Rod-Shaped Secondary Building Units
Abstract
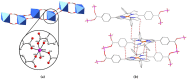
Metal-organic frameworks (MOFs) encompass a rapidly expanding class of materials with diverse potential applications including gas storage, molecular separation, sensing and catalysis. So-called 'rod MOFs', which comprise infinitely extended 1D secondary building units (SBUs), represent an underexplored subclass of MOF. Further, porphyrins are considered privileged ligands for MOF synthesis due to their tunable redox and photophysical properties. In this study, the CuII complex of 5,15-bis(4-carboxyphenyl)-10,20-diphenylporphyrin (H2L-CuII, where H2 refers to the ligand's carboxyl H atoms) is used to prepare two new 2D porphyrinic rod MOFs PROD-1 and PROD-2. Single-crystal X-ray analysis reveals that these frameworks feature 1D MnII- or CoII-based rod-like SBUs that are coordinated by labile solvent molecules and photoactive porphyrin moieties. Both materials were characterised using infrared (IR) spectroscopy, powder X-ray diffraction (PXRD) spectroscopy and thermogravimetric analysis (TGA). The structural attributes of PROD-1 and PROD-2 render them promising materials for future photocatalytic investigations.
Keywords: 2D MOF; 2D materials; MOF; Porphyrin MOF; Porphyrinoids; coordination chemistry; metal-organic framework; rod MOF.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Cheong V.F., Moh P.Y. Recent Advancement in Metal–Organic Framework: Synthesis, Activation, Functionalisation, and Bulk Production. Mater. Sci. Technol. 2018;34:1025–1045. doi: 10.1080/02670836.2018.1468653. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

